– In this topic, we will discuss The Physical and Chemical properties of Matter and The Physical and Chemical Changes. Physical and Chemical properties – To distinguish among samples of different kinds of matter, we determine and compare their properties. – We recognize different kinds of matter by their properties, …
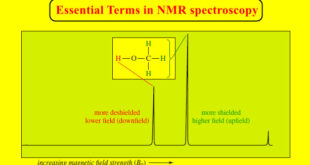
Read More »NMR – Essential Terms in NMR spectroscopy
– In this topic, we will discuss some Essential Terms in NMR spectroscopy. Essential Terms in NMR spectroscopy accidentally equivalent nuclei – Nuclei that are not chemically equivalent, yet absorb at nearly the same chemical shift and are not resolved. – Nuclei that absorb at the same chemical shift cannot …
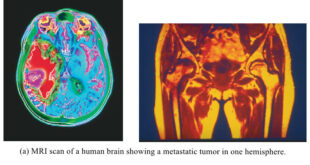
Read More »Nuclear Magnetic Resonance Imaging – NMR imaging
– In this topic, we will discuss Nuclear Magnetic Resonance Imaging. Nuclear magnetic resonance imaging – When chemists use NMR spectroscopy, they take great pains to get the most uniform magnetic field possible (often homogeneous to within one part per billion). – They place small tubes of homogeneous solutions in …
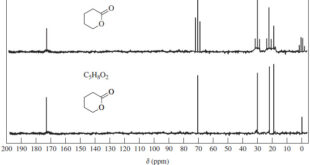
Read More »Interpreting Carbon NMR Spectra
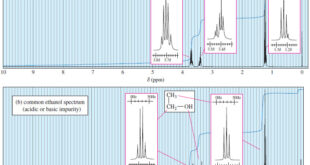
Interpreting Carbon NMR Spectra – Interpreting Carbon NMR Spectra (13C NMR spectra) uses the same principles as interpreting 1H NMR spectra. – In fact, carbon spectra are often easier to interpret. – The 13C NMR spectrum provides the following information: (1) The number of different signals implies how many different …
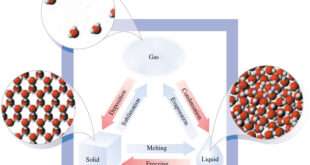
Read More »Solids, liquids, and gases.
What’s the difference between Solids, liquids, and gases? – It is easy to tell the difference between solids, liquids and gases – A solid has a fixed shape and a fixed volume. It does not flow. Think of all the solid things around you: their shapes and volumes do not …
Read More »Properties of Amino acids – Acidic and Basic Properties
Acidic and Basic Properties of Amino acids – Amino acids in aqueous solution contain weakly acidic α-carboxyl groups and weakly basic α-amino groups. – In addition, each of the acidic and basic amino acids contains an ionizable group in its side chain. – Thus, both free amino acids and some …
Read More »Matter and Energy
Definition of Matter and Energy What is Matter? – Matter is anything that has mass and occupies space. – Mass is a measure of the quantity of matter in a sample of any material. – The more massive an object is, the more force is required to put it in …
Read More »Amino acids – Structure of Amino acids
Structure of Amino acids – Although more than 300 different amino acids have been described in nature, only 20 are commonly found as constituents of mammalian proteins. – Each amino acid (except for proline, which has a secondary amino group) has a carboxyl group, a primary amino group, and a …
Read More »Carbon-13 NMR Spectroscopy
– In this topic, we will discuss The Carbon-13 NMR Spectroscopy. Carbon-13 NMR Spectroscopy – Where does a carbonyl group absorb in the NMR? Where does an internal alkyne absorb? – In the proton NMR, both of these groups are invisible. Sometimes we can infer their presence: If the carbonyl …
Read More »The Formation of Complexes
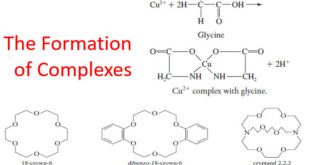
The Formation of Complexes – Most metal ions react with electron-pair donors to form coordination compounds or complexes. – The donor species, or ligand, must have at least one pair of unshared electrons available for bond formation. – Water, ammonia, and halide ions are common inorganic ligands. In fact most …
Read More »Gels : Defination, Types, Properties
– In this topic, we will discuss The Gels : Defination, Types, and Properties. What are Gels? – A gel is a jelly-like colloidal system in which a liquid is dispersed in a solid medium. – For example, when a warm sol of gelatin is cooled, it sets to a …
Read More »Emulsions : Defination, Types, Examples, Preparation
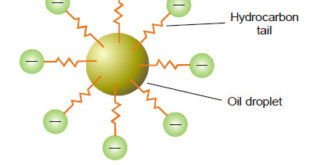
Defination of Emulsions – Emulsions are liquid-liquid colloidal systems. – In other words, an emulsion may be defined as a dispersion of finely divided liquid droplets in another liquid. – Generally one of the two liquids is water and the other, which is immiscible with water, is designated as oil. …
Read More »Time Dependence of NMR Spectroscopy
– In this topic, we will discuss The Time Dependence of NMR Spectroscopy. Time Dependence of NMR Spectroscopy – We have already seen evidence that NMR does not provide an instantaneous picture of a molecule. – For example, a terminal alkyne does not give a spectrum where the molecules oriented …
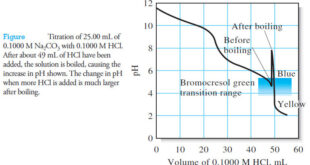
Read More »Applications of Neutralization Titrations
– In this topic, we will discuss The Applications of Neutralization Titrations. Typical Applications of Neutralization Titrations – Neutralization titrations are used to determine the many inorganic, organic, and biological species that possess acidic or basic properties. – In addition, however, there are nearly as many applications in which the …
Read More »Stereochemical Nonequivalence of Protons in NMR Spectroscopy
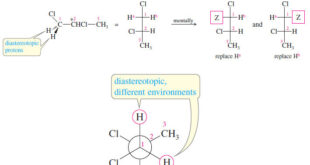
– In the this topic we talk about Stereochemical Nonequivalence of Protons in NMR Spectroscopy. Stereochemical Nonequivalence of Protons – Stereochemical differences often result in different chemical shifts for protons on the same carbon atom. – For example, the two protons on C1 of allyl bromide (3-bromopropene) are not equivalent. …
Read More »Associated Colloids
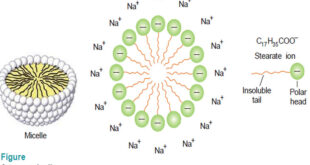
– In this subject, we will discuss the Associated Colloids. Associated Colloids – The molecules of substances as soaps and artificial detergents are smaller than the colloidal particles. However in concentrated solutions these molecules form aggregates of colloidal size. – Substances whose molecules aggregate spontaneously in a given solvent to …
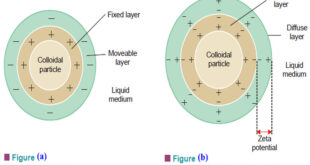
Read More »Electrical Properties of Sols
Electrical Properties of Sols – In this topic we will discuss the Electrical Properties of Sols as follow: (1) The sol particles carry an electric charge. (2) Electrophoresis. (3) Electro-osmosis. (4) Coagulation or Precipitation. (5) Protective action of sols. (6) Origin of charge on sol particles. (1) The sol particles …
Read More »Reagents for Neutralization Titrations
– In this subject, we will discuss the Reagents for Neutralization Titrations. Reagents for Neutralization Titrations – we noted before that strong acids and strong bases produce the largest change in pH at the equivalence point. – For this reason, standard solutions for neutralization titrations are always prepared from these …
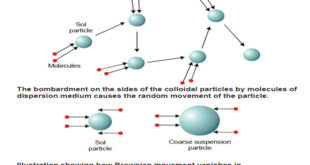
Read More »Kinetic Properties of Sols – Brownian movement
Kinetic Properties of Sols – In this topic, we will discuss Kinetic Properties of Sols. Brownian Movement – When a sol is examined with an ultramicroscope, the suspended particles are seen as shining specks of light. – By following an individual particle it is observed that the particle is undergoing …
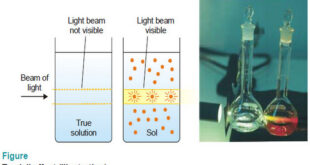
Read More »Optical Properties of Sols
Optical Properties of Sols – In this topic, we will discuss Optical Properties of Sols as follow: Sols exhibit Tyndall effect Ultramicroscope shows up the presence of individual particles Sol particles can be seen with an Electron microscope (1) Sols exhibit Tyndall effect – When a strong beam of light …
Read More » Read Chemistry
Read Chemistry