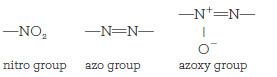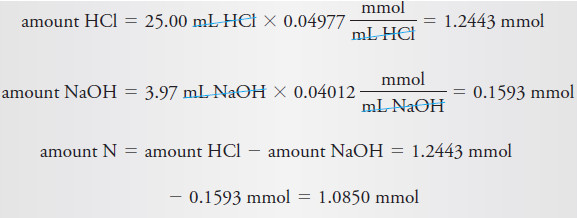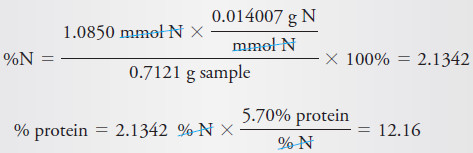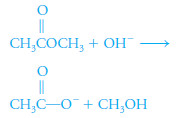Applications of Neutralization Titrations
– In this topic, we will discuss The Applications of Neutralization Titrations.
Typical Applications of Neutralization Titrations
– Neutralization titrations are used to determine the many inorganic, organic, and biological species that possess acidic or basic properties.
– In addition, however, there are nearly as many applications in which the analyte is converted to an acid or base by suitable chemical treatment and then titrated with a standard strong base or acid.
– There are two major types of end points that are widely used in neutralization titrations.
– The first is a visual end point based on indicators.
– The second is a potentiometric end point in which the potential of a glass/calomel electrode system is determined with a pH meter or another voltagemeasuring device.
– The measured voltage is directly proportional to pH.
(1) Elemental Analysis
– Several important elements that occur in organic and biological systems are conveniently determined by methods that have an acid/base titration as the final step.
– Generally, the elements susceptible to this type of analysis are nonmetals such as carbon, nitrogen, chlorine, bromine, fluorine, as well as a few other less common species.
– Pretreatment converts the element to an inorganic acid or base that is then titrated.
– A few examples follow.
Nitrogen
– Nitrogen occurs in a wide variety of substances of interest in the life sciences, in industry, and in agriculture.
– Examples include amino acids, proteins, synthetic drugs, fertilizers, explosives, soils, potable water supplies, and dyes.
– Thus, analytical methods for the determination of nitrogen, particularly in organic substrates, are extremely important.
– The most common method for determining organic nitrogen is the Kjeldahl method, which is based on a neutralization titrations.
– The procedure is straightforward, requires no special equipment, and is readily adapted to the routine analysis of large numbers of samples.
– The Kjeldahl method, or one of its modifications, is the standard process for determining the protein content of grains, meats, and biological materials.
– Since most proteins contain approximately the same percentage of nitrogen, multiplication of this percentage by a suitable factor (6.25 for meats, 6.38 for dairy products, and 5.70 for cereals) gives the percentage of protein in a sample.
– In the Kjeldahl method, the sample is decomposed in hot, concentrated sulfuric acid to convert the bound nitrogen to ammonium ion.
– The resulting solution is then cooled, diluted, and made basic, a process that converts the ammonium ions to ammonia.
– The ammonia is distilled from the basic solution, collected in an acidic solution, and determined by a neutralization titrations.
– The critical step in the Kjeldahl method is the decomposition with sulfuric acid, which oxidizes the carbon and hydrogen in the sample to carbon dioxide and water.
– The fate of the nitrogen, however, depends on its state of combination in the original sample.
– Amine and amide nitrogens are quantitatively converted to ammonium ion.
– In contrast, nitro, azo, and azoxy groups are likely to yield the element or its various oxides, all of which are lost from the hot acidic medium. This loss can be avoided by pretreating the sample with a reducing agent to form amides or amines.
– In one such prereduction scheme, salicylic acid and sodium thiosulfate are added to the concentrated sulfuric acid solution containing the sample.
– After a brief period, the digestion is performed in the usual way.
– Pyridine, pyridine derivatives, and some other aromatic heterocyclic compounds are particularly resistant to complete decomposition by sulfuric acid.
– Such compounds yield low results as a consequence unless special precautions are taken.
– The decomposition step is frequently the most time-consuming aspect of a Kjeldahl determination.
– Some samples may require heating periods in excess of 1 hr.
– Numerous modifications of the original procedure have been proposed with the aim of shortening the digestion time.
– In the most widely used modification, a neutral salt, such as potassium sulfate, is added to increase the boiling point of the sulfuric acid solution and thus the temperature at which the decomposition occurs.
– In another modification, a solution of hydrogen peroxide is added to the mixture after the digestion has decomposed most of the organic matrix.
– Many substances catalyze the decomposition of organic compounds by sulfuric acid. Mercury, copper, and selenium, either combined or in the elemental state, are effective.
– Mercury(II), if present, must be precipitated with hydrogen sulfide prior to distillation to prevent retention of ammonia as a mercury(II) ammine complex.
– Example (1) illustrates the calculations used in the Kjeldahl method.
Example (1)
A 0.7121 g sample of wheat flour was analyzed by the Kjeldahl method. The
ammonia formed by addition of concentrated base after digestion with H2SO4 was
distilled into 25.00 mL of 0.04977 M HCl. The excess HCl was then back-titrated
with 3.97 mL of 0.04012 M NaOH. Calculate the percent protein in the flour, using
the 5.70 factor for cereal.
Solution:
Sulfur
– Sulfur in organic and biological materials is conveniently determined by burning the sample in a stream of oxygen.
– The sulfur dioxide (as well as the sulfur trioxide) formed during the oxidation is collected by distillation into a dilute solution of hydrogen peroxide:
SO2 (g) + H2O2 → H2SO4
– The sulfuric acid is then titrated with standard base.
Other Elements
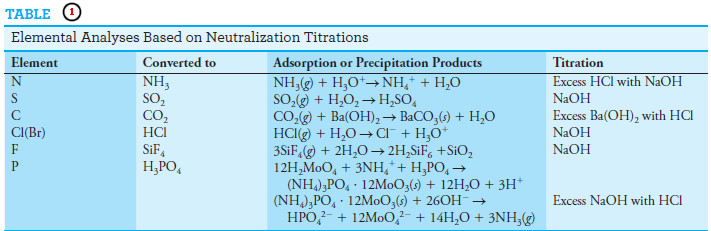
– Table (1) lists other elements that can be determined by neutralization methods.
(2) The Determination of Inorganic Substances
– Various inorganic species can be determined by titration with strong acids or bases.
– A few examples follow.
Ammonium Salts
– Ammonium salts are conveniently determined by conversion to ammonia with strong base followed by distillation.
– The ammonia is collected and titrated as in the Kjeldahl method.
Nitrates and Nitrites
– The method just described for ammonium salts can be extended to the determination of inorganic nitrate or nitrite.
– These ions are first reduced to ammonium ion by reaction with an alloy of 50% Cu, 45% Al, and 5% Zn(Devarda’s alloy).
– Granules of the alloy are introduced into a strongly alkaline solution of the sample in a Kjeldahl flask.
– The ammonia is distilled after reaction is complete. An alloy of 60% Cu and 40% Mg (Arnd’s alloy) has also been used as the reducing agent.
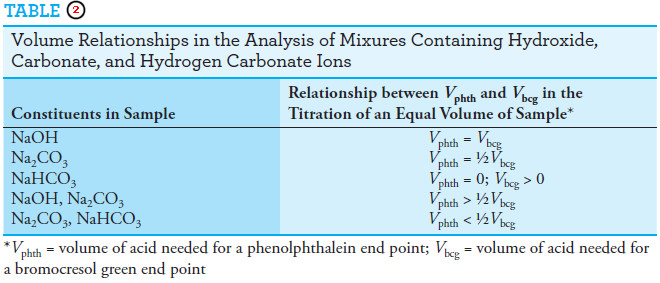
Carbonate and Carbonate Mixtures
– The qualitative and quantitative determination of the constituents in a solution containing sodium carbonate, sodium hydrogen carbonate, and sodium hydroxide, either alone or as various mixtures, provides interesting examples of how neutralization titrations can be applied to analyze mixtures.
– No more than two of these three constituents can exist in appreciable amount in any solution because reaction eliminates the third.
– For example, mixing sodium hydroxide with sodium hydrogen carbonate results in the formation of sodium carbonate until one or the other (or both) of the original reactants is exhausted.
– If the sodium hydroxide is used up, the solution will contain sodium carbonate and sodium hydrogen carbonate.
– If sodium hydrogen carbonate is depleted, sodium carbonate and sodium hydroxide will remain.
– but If equimolar amounts of sodium hydrogen carbonate and sodium hydroxide are mixed, the principal solute species will be sodium carbonate.
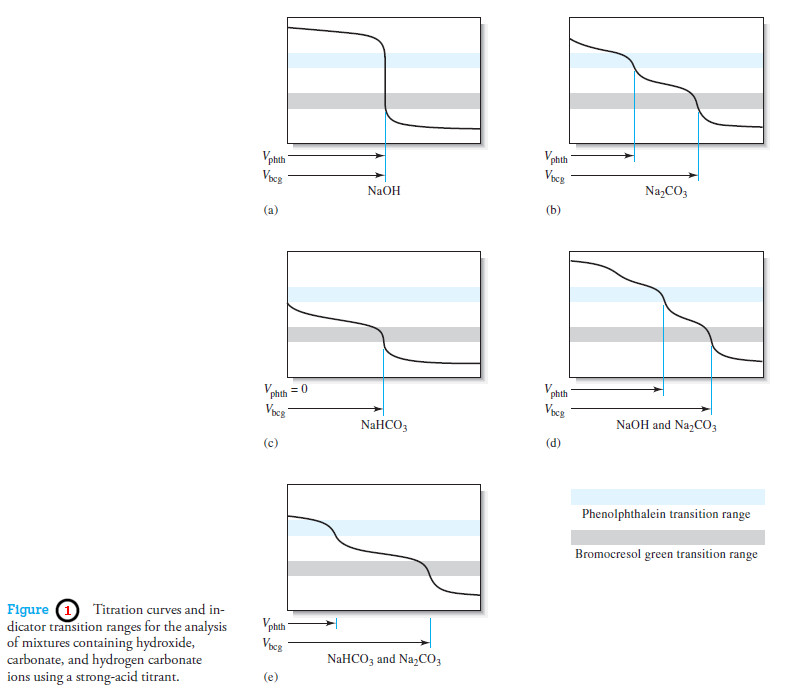
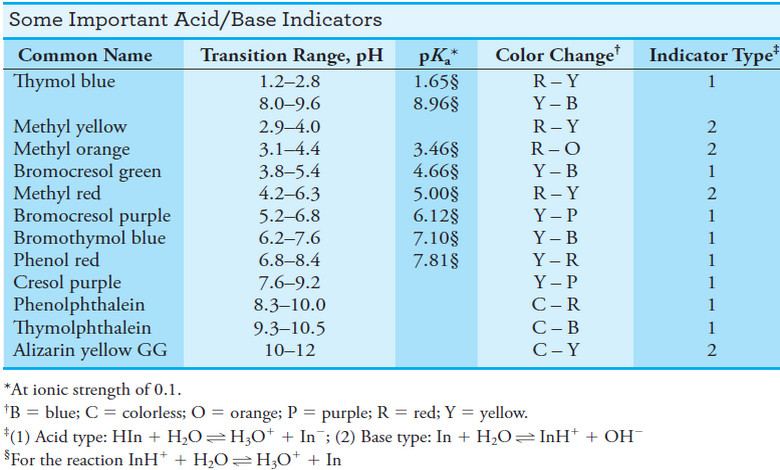
– The analysis of such mixtures requires two titrations with a strong acid: one using an alkaline-range indicator, such as phenolphthalein, and the other with an acid-range indicator, such as bromocresol green.
– The composition of the solution can then be deduced from the relative volumes of acid needed to titrate equal volumes of the sample (see Table (2) and Figure 1).
– Once the composition of the solution has been established, the volume data can be used to determine the concentration of each component in the sample.
– The accuracy of methods for analyzing solutions containing mixtures of carbonate and hydrogen carbonate ions or carbonate and hydroxide ions can be greatly improved by taking advantage of the limited solubility of barium carbonate in neutral and basic solutions.
– For example, in the Winkler method for the analysis of carbonate/hydroxide mixtures, both components are titrated with a standard acid to the end point with an acid-range indicator, such as bromocresol green (the end point being established after the solution is boiled to remove carbon dioxide).
– An unmeasured excess of neutral barium chloride is then added to a second aliquot of the sample solution to precipitate the carbonate ion, following which the hydroxide ion is titrated to a phenolphthalein end point.
– The presence of the sparingly soluble barium carbonate does not interfere as long as the concentration of barium ion is greater than 0.1 M.
– Carbonate and hydrogen carbonate ions can be accurately determined in mixtures by first titrating both ions with standard acid to an end point with an acid-range indicator (with boiling to eliminate carbon dioxide).
– The hydrogen carbonate in a second aliquot is converted to carbonate by the addition of a known excess of standard base.
– After a large excess of barium chloride has been introduced, the excess base is titrated with standard acid to a phenolphthalein end point.
– The presence of solid barium carbonate does not influence end-point detection in either of these methods.
(3) The Determination of Organic Functional Groups
– Neutralization titrations provide convenient methods for the direct or indirect determination of several organic functional groups.
– Brief descriptions of methods for the more common groups follow.
Carboxylic and Sulfonic Acid Groups
– Carboxylic and sulfonic acids are very common organic acids.
– Most carboxylic acids have dissociation constants that range between 10–4 and 10–6, and thus, these compounds are readily titrated.
– An indicator that changes color in a basic range, such as phenolphthalein, is required.
– Many carboxylic acids are not sufficiently soluble in water to permit direct titration in aqueous solution.
– When this problem exists, the acid can be dissolved in ethanol and titrated with aqueous base.
– Alternatively, the acid can be dissolved in an excess of standard base followed by back-titration with standard acid.
– Sulfonic acids are generally strong acids that easily dissolve in water.
– Titration with standard base can be used for the determination.
– Neutralization titrations are often used to determine the equivalent masses of purified organic acids.
– Equivalent masses serve as an aid in the qualitative identification of organic acids.
Amine Groups
– Aliphatic amines generally have base dissociation constants on the order of 10-5 and can be titrated directly with a solution of strong acid.
– Aromatic amines such as aniline and its derivatives, however, are usually too weak for titration in aqueous solutions (Kb = 10–10).
– Likewise, cyclic amines with aromatic character, such as pyridine and its derivatives, are usually too weak for titration in aqueous solutions.
– Many saturated cyclic amines, such as piperdine, tend to resemble aliphatic amines in their acid/base behavior and can thus be titrated in aqueous media.
– Many amines that are too weak to be titrated as bases in water are easily titrated in nonaqueous solvents, such as anhydrous acetic acid, which enhance their basicity.
Ester Groups
– Esters are commonly determined by saponification with a measured quantity of standard base:
R1COOR2 + OH- → R1COO- + HOR2
– The excess base is then titrated with standard acid.
– Esters vary widely in their rates of saponification.
– Some require several hours of heating with a base to complete the process, while a few react rapidly enough that direct titration with standard base is feasible.
– Typically, the ester is refluxed with standard 0.5 M KOH for 1 to 2 hours.
– After cooling, the excess base is titrated with standard acid.
– Saponification is the process by which an ester is hydrolyzed in alkaline solution to give an alcohol and a conjugate base. For example,
Hydroxyl Groups
– Hydroxyl groups in organic compounds can be determined by esterification with various carboxylic acid anhydrides or chlorides.
– The two most common reagents are acetic anhydride and phthalic anhydride. With acetic anhydride, the reaction is
(CH3CO)2O + ROH → CH3COOR + CH3COOH
– Usually, the sample is mixed with a carefully measured volume of acetic anhydride in
pyridine. After heating, water is added to hydrolyze the unreacted anhydride according to
(CH3CO)2O + H2O → 2CH3COOH
– The acetic acid is then titrated with a standard solution of alcoholic sodium or potassium hydroxide.
– A blank is carried through the analysis to establish the original amount of anhydride.
– Amines, if present, are converted quantitatively to amides by acetic anhydride.
– A correction for this potential interference is frequently made by direct titration of another portion of the sample with standard acid.
Carbonyl Groups
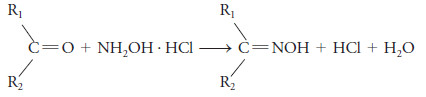
– Many aldehydes and ketones can be determined with a solution of hydroxylamine hydrochloride.
– The reaction, which produces an oxime, is
where R2 may be hydrogen.
– The liberated HCl is titrated with base.
– Once again, the conditions necessary for quantitative reaction vary.
– Typically, 30 minutes suffices for aldehydes, while many ketones require refluxing with the reagent for 1 hour or more.
(4) The Determination of Salts
– The total salt content of a solution can be determined accurately by acid/base titration.
– The salt is converted to an equivalent amount of an acid or base by passing a solution containing the salt through a column packed with an ion-exchange resin. (This application is considered in more detail in Section 31D.)
– Standard acid or base solutions can also be prepared with ion-exchange resins.
– A solution containing a known mass of a pure compound, such as sodium chloride, is washed through the resin column and diluted to a known volume.
– The salt releases an equivalent amount of acid or base from the resin.
– The concentration of the acid or base can then be calculated from the known mass of the original salt.
Reference
- Modern analytical chemistry / David Harvey / The McGraw-Hill Companies, Inc./ , 2000 . USA
- Dean’s Analytical Chemistry Handbook / Pradyot Patnaik / The McGraw-Hill Companies, 2nd Editionm, 2004 .USA
- Fundamentals of analytical chemistry / Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch. (ninth edition) , 2014 . USA