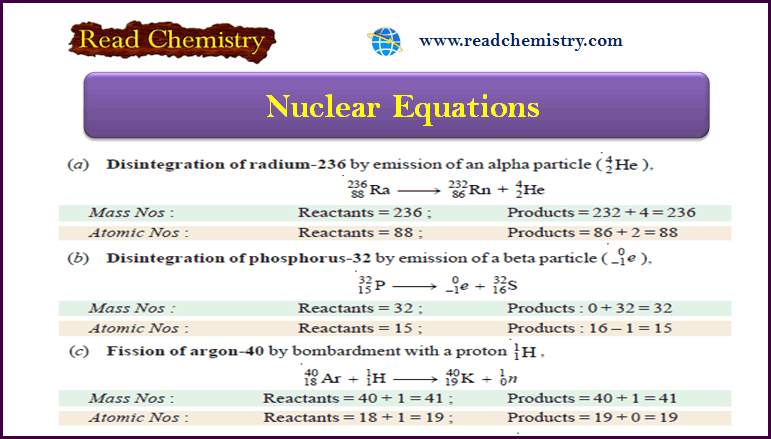
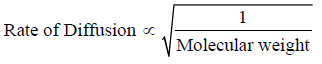Separation of Isotopes
– In this subject, we will discuss the Separation of Isotopes by seven methods
Separation of Isotopes
– Since isotopes have exactly similar chemical properties, their separation by chemical means is out of the question.
– Their difference in those physical properties which depend on the mass of the atom has been utilized to effect their separation.
– The methods commonly employed for Separation of Isotopes are:
(1) Gaseous Diffusion
(2) Thermal Diffusion
(3) Distillation
(4) Ultracentrifuge
(5) Electromagnetic Separation
(6) Fractional Electrolysis
(7) Laser Separation
(1) Gaseous Diffusion
– The rate of diffusion of a gas is inversely proportional to the square root of the molecular weight (Graham’s Law of Diffusion).
– Thus when a mixture of two gaseous isotopes is allowed to diffuse through a porous partition, the lighter isotope passes through more rapidly than the heavier one.
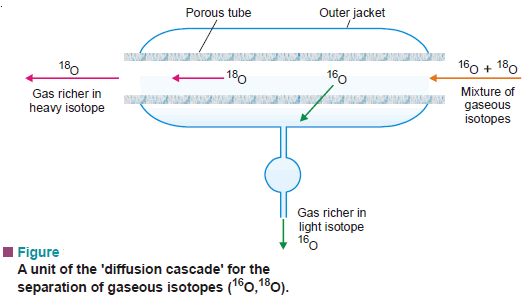
– The isotopes of neon (20Ne, 22Ne) and oxygen (16O, 18O) were separated by this method.
– The mixture of gaseous isotopes is passed through a porous tube sealed in an outer jacket (see Fig below).
– The lighter isotope passes into the jacket, while the residual gas becomes richer in the heavier isotope.
– In actual practice, a cascade of many ‘Diffusion units’ is used to achieve an appreciable separation.
– This process has been recently used for the separation of the gaseous fluorides 235UF6 and 238UF6.
– It provides a procedure for the effective separation of the isotopes of uranium, namely, U-238 and U-235 (needed for atomic energy).
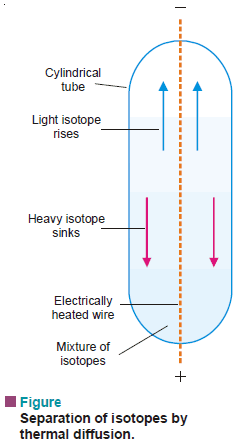
(2) Thermal Diffusion
– A long vertical tube with an electrically heated wire running down its axis is used.
– When a mixture of gaseous isotopes is introduced into the tube, the lighter particles diffuse more rapidly to the central hot region.
– Here they are carried upwards by convection currents.
– The heavier particles, on the other hand, travel to the cooler inner surface of the tube and sink to the bottom.
– Thus the lighter isotope collects at the top and the heavier one at the bottom.
– The isotopes of chlorine Cl-35 and Cl-37, have been separated by this process.
– The fluorides of uranium have also been separated by thermal diffusion.
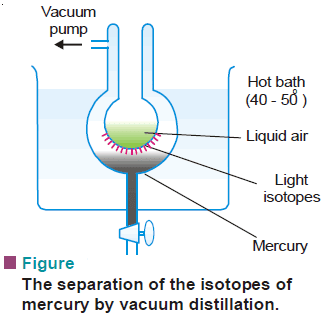
(3) Distillation
– The lighter isotope will be distilled over first, leaving the heavier one behind.
– The isotopes of mercury were separated by this method.
– The frozen mercury from the cooled surface is removed, melted, and evaporated under vacuum again.
– The whole process is repeated several times to separate the isotopes of mercury.
(4) Ultracentrifuge
– The mixture of isotopes is rotated in a high-speed centrifuge.
– The heavier isotope is concentrated at the periphery.
– The separation depends essentially on the molecular mass and not its square root, causing better separation.
– The gaseous fluorides of U-235 and U-238 have been separated by this method.
(5) Electromagnetic Separation
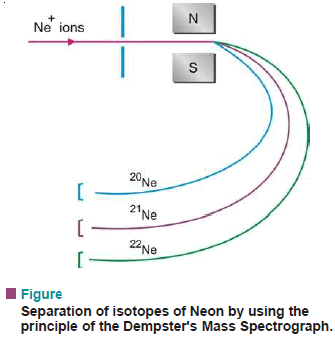
– This method uses the principle of the Mass Spectrograph (Dempster).
– For example, the beam of ions of the isotopes of neon (Ne-20, Ne-21, Ne-22) as obtained in the mass spectrograph, is then passed between the poles of a magnet.
– The different isotopes are deflected to different extents and are collected in cooled chambers placed in appropriate positions.
– Although the quantities obtained by this method are very small indeed, the separation is complete.
(6) Fractional Electrolysis
– Here the principle is that the rates of liberation of the isotopes of an element at an electrode during electrolysis are different.
– This is so because the ions of the heavier isotope move slower, while those of the lighter isotope move faster to the opposite electrode.
– Urey (1931) separated the two isotopes of hydrogen, H-1, and H-2, by the electrolysis of acidified water.
– H-1 (protium) is liberated five times as rapidly as H-2 (deuterium) at the cathode.
– The residual water becomes richer in heavy water or deuterium oxide 2H2O or D2O which upon further electrolysis yields gas richer in deuterium
(7) Laser Separation
– A laser is a very fine beam of electromagnetic radiation that consists of photons corresponding to a single wavelength, frequency, or energy.
– All the waves in the beam are in phase with all troughs and peaks moving through space together.
– In recent years, the development of lasers has been used for the separation of isotopes.
– If the laser light is of the appropriate wavelength, one isotope will absorb the energy, while another isotope will not.
– The slight difference in absorption spectra of the two isotopes thus produced has been used to separate the more energetic isotope from the other.
– The laser method has been used successfully for the separation of isotopes of chlorine and sulfur.
– Potentially, laser isotope separation of uranium is 1000 times more efficient than gaseous diffusion separation.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.