Nomenclature of Molecular Compounds
How to Name Molecular Compounds
– Unlike ionic compounds, Molecular compounds contain discrete molecular units.
– They are usually composed of nonmetallic elements.
– Many molecular compounds are binary compounds.
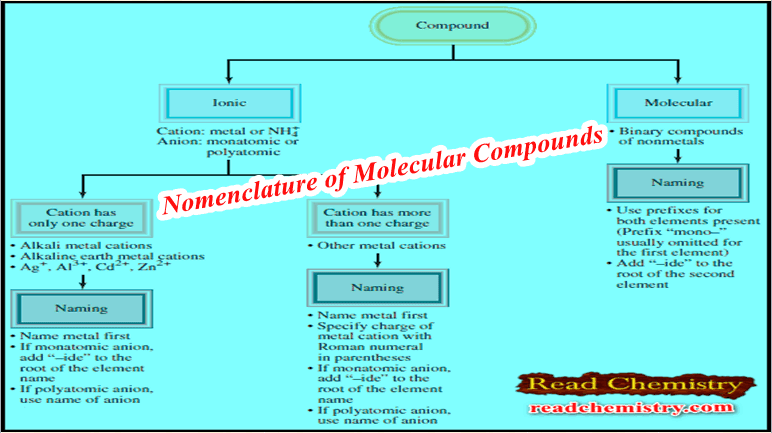
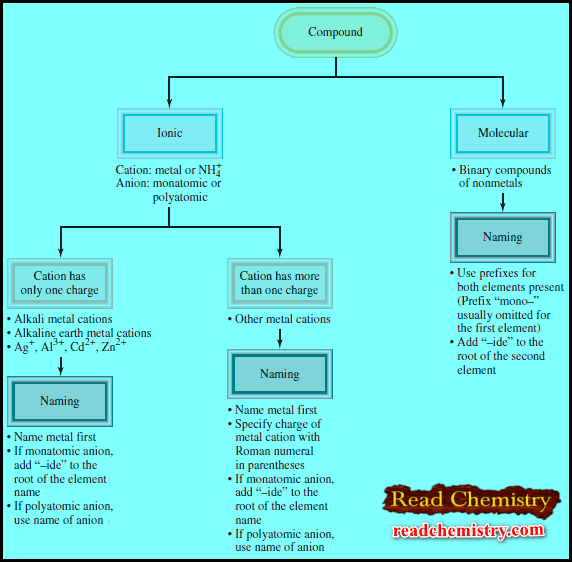
– Naming binary molecular compounds is similar to naming binary ionic compounds.
– We place the name of the first element in the formula first, and the second element is named by adding “-ide” to the root of the element name.
– Some examples are:
HCl Hydrogen chloride
SiC Silicon carbide
HBr Hydrogen bromide
– It is quite common for one pair of elements to form several different compounds.
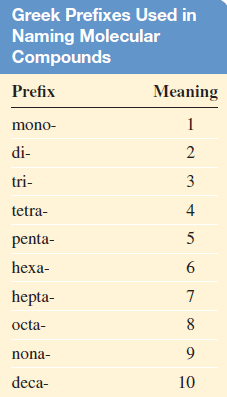
– In these cases, confusion in naming the compounds is avoided by the use of Greek prefixes to denote the number of atoms of each element present (see Table).
– Consider these examples:
CO Carbon monoxide
SO3 Sulfur trioxide
CO2 Carbon dioxide
NO2 Nitrogen dioxide
SO2 Sulfur dioxide
N2O4 Dinitrogen tetroxide
A guideline for naming Molecular compounds with prefixes
– These guidelines are helpful when you are naming compounds with prefixes:
– The prefix “mono-” may be omitted for the first element.
– For example, PCl3 is named phosphorus trichloride, not monophosphorus trichloride.
– Thus, the absence of a prefix for the first element usually means that only one atom of that element is present in the molecule.
– For oxides, the ending “a” in the prefix is sometimes omitted.
– For example, N2O4 may be called dinitrogen tetroxide rather than dinitrogen tetraoxide.
– Exceptions to the use of Greek prefixes are molecular compounds containing hydrogen.
– Traditionally, many of these compounds are called either by their common, non-systematic names or by names that do not specifically indicate the number of H atoms present:
B2H6 Diborane
PH3 Phosphine
CH4 Methane
H2O Water
SiH4 Silane
H2S Hydrogen sulfide
NH3 Ammonia
– Note that even the order of writing the elements in the formulas is irregular.
– These examples show that H is written first in water and hydrogen sulfide, whereas H is written last in the other compounds.
– Writing formulas for molecular compounds is usually straightforward.
– Thus, the name arsenic trifluoride means that there is one As atom and three F atoms in each molecule and the molecular formula is AsF3.
– Note that the order of elements in the formula is the same as that in its name.
– The figure below summarizes the steps for naming ionic and molecular compounds:
Solved Problems
Example (1): Name the following these compounds:
(a) PBr5
(b) As2O5
Solution
(a) Because there are five bromine atoms present, the compound is phosphorus pentabromide.
(b) There are two arsenic atoms and five oxygen atoms present, so the compound is diarsenic pentoxide.
– Note that the “a” is omitted in “Penta.
Example (1): Write chemical formulas for the following molecular compounds:
(a) bromine trifluoride
(b) diboron trioxide
Solution
(a) Because there are three fluorine atoms and one bromine atom present, the formula is BrF3.
(b) There are two boron atoms and three oxygen atoms present, so the formula is B2O3.
Reference: General Chemistry: The Essential Concepts / Raymond Chang, Jason Overby. (sixth edition) .