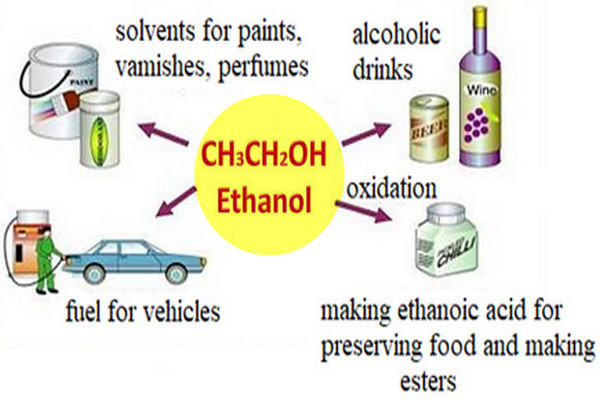
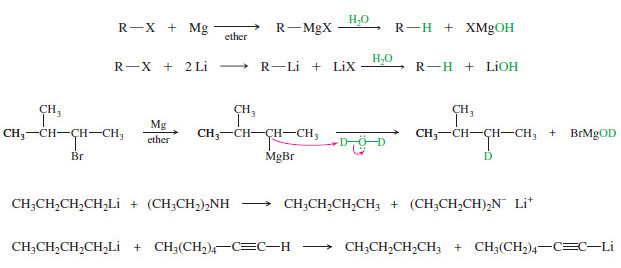
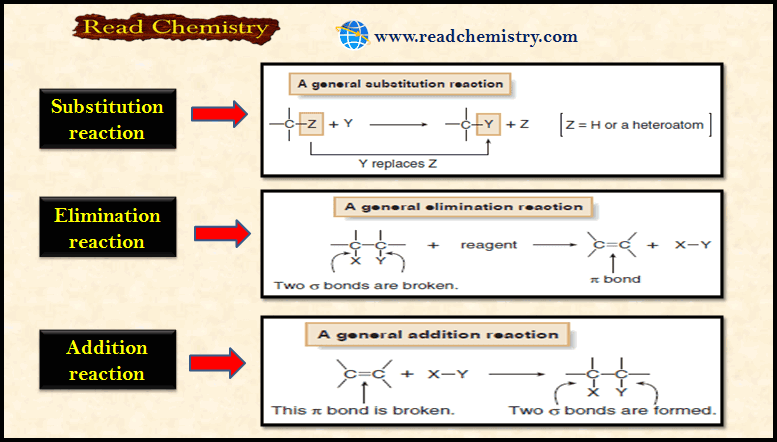
Organic Chemistry
Important concepts in Hybridization That Come from Quantum Mechanics
Important concepts in Hybridization
(1) An atomic orbital (AO) corresponds to a region of space about the nucleus of a single atom where there is a high probability of finding an electron. Atomic orbitals called s orbitals are spherical; those called p orbitals are like two almost-tangent spheres. Orbitals can hold a maximum of two electrons when their spins are paired. Orbitals are described by the square of a wave function, ψ2, and each orbital has a characteristic energy. The phase signs associated with an orbital may be + or -.
(2) When atomic orbitals overlap, they combine to form molecular orbitals (MOs). Molecular orbitals correspond to regions of space encompassing two (or more) nuclei where electrons are to be found. Like atomic orbitals, molecular orbitals can hold up to two electrons if their spins are paired.
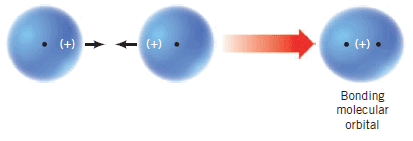
(3) When atomic orbitals with the same phase sign interact, they combine to form a bonding molecular orbital:
The electron probability density of a bonding molecular orbital is large in the region of space between the two nuclei where the negative electrons hold the positive nuclei together.
(4) An antibonding molecular orbital forms when orbitals of opposite phase sign overlap:
An antibonding orbital has higher energy than a bonding orbital. The electron probability density of the region between the nuclei is small and it contains a node – a region where ψ = 0. Thus, having electrons in an antibonding orbital does not help hold the nuclei together. The internuclear repulsions tend to make them fly apart.
(5) The energy of electrons in a bonding molecular orbital is less than the energy of the electrons in their separate atomic orbitals. The energy of electrons in an antibonding orbital is greater than that of electrons in their separate atomic orbitals.
(6) The number of molecular orbitals always equals the number of atomic orbitals from which they are formed. Combining two atomic orbitals will always yield two molecular orbitals—one bonding and one antibonding.
(7) Hybrid atomic orbitals are obtained by mixing (hybridizing) the wave functions for orbitals of different types (i.e., s and p orbitals) but from the same atom.
(8) Hybridizing three p orbitals with one s orbital yields four sp3 orbitals. Atoms that are sp3 hybridized direct the axes of their four sp3 orbitals toward the corners of a tetrahedron. The carbon of methane is sp3 hybridized and tetrahedral.
(9) Hybridizing two p orbitals with one s orbital yields three sp2 orbitals. Atoms that are sp2 hybridized point the axes of their three sp2 orbitals toward the corners of an equilateral triangle. The carbon atoms of ethene are sp2 hybridized and trigonal planar.
(10) Hybridizing one p orbital with one s orbital yields two sp orbitals. Atoms that are sp hybridized orient the axes of their two sp orbitals in opposite directions (at an angle of 180o). The carbon atoms of ethyne are sp hybridized and ethyne is a linear molecule.
(11) A sigma (σ) bond (a type of single bond) is one in which the electron density has circular symmetry when viewed along the bond axis. In general, the skeletons of organic molecules are constructed of atoms linked by sigma bonds.
(12) A pi (π) bond, part of double and triple carbon–carbon bonds, is one in which the electron densities of two adjacent parallel p orbitals overlap sideways to form a bonding pi molecular orbital.
Reference: Organic chemistry / T.W. Graham Solomons , Craig B.Fryhle , Scott A.snyder , / ( eleventh edition) / 2014.