Organic Chemistry
The Structure of Ethyne (Acetylene): sp Hybridization
The Structure of Ethyne (Acetylene): sp Hybridization
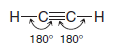
** Hydrocarbons in which two carbon atoms share three pairs of electrons between them, and are thus bonded by a triple bond, are called alkynes. The two simplest alkynes are ethyne and propyne.
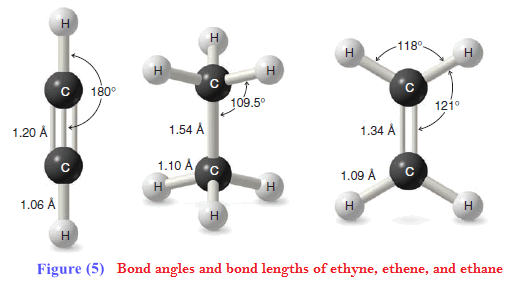
** Ethyne, a compound that is also called acetylene, consists of a linear arrangement of atoms. The H-C≡ C bond angles of ethyne molecules are 180o
** We can account for the structure of ethyne on the basis of orbital hybridization as we did for ethane and ethene. In our model for ethane we saw that the carbon orbitals are sp3hybridized, and in our model for ethene we saw that they are sp2 hybridized. In our model for ethyne we shall see that the carbon atoms are sp hybridized.
** The mathematical process for obtaining the sp hybrid orbitals of ethyne can be visualized
in the following way (Fig. 1).
 |
| Figure (1) |
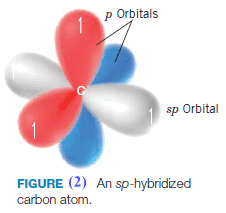
(1) The 2s orbital and one 2p orbital of carbon are hybridized to form two sp orbitals.
(2) The remaining two 2p orbitals are not hybridized.
** Calculations show that the sp hybrid orbitals have their large positive lobes oriented at an angle of 180owith respect to each other. The two 2p orbitals that were not hybridized are each perpendicular to the axis that passes through the center of the two sp orbitals (Fig. 2). We place one electron in each orbital.
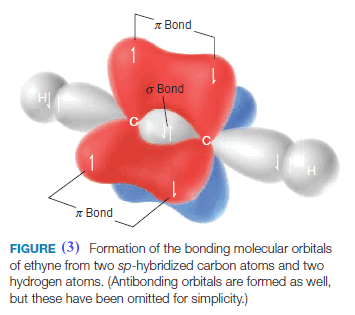
** We envision the bonding molecular orbitals of ethyne being formed in the following way (Fig. 3).
(3) Two carbon atoms overlap sp orbitals to form a sigma bond between them (this is one bond of the triple bond). The remaining two sp orbitals at each carbon atom overlap with s orbitals from hydrogen atoms to produce two sigma C-H bonds.
(4) The two p orbitals on each carbon atom also overlap side to side to form two p bonds. These are the other two bonds of the triple bond.
(5) The carbon–carbon triple bond consists of two π bonds and one σ bond.
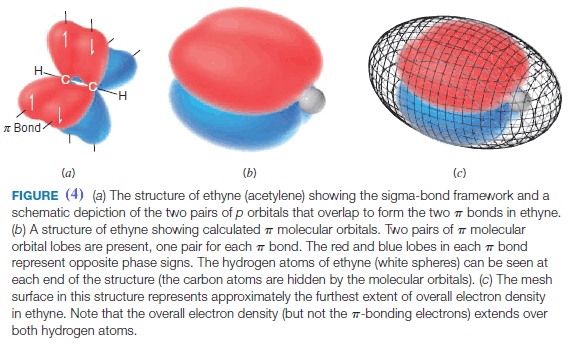
** Structures for ethyne based on calculated molecular orbitals and electron density are shown in Fig. 4. Circular symmetry exists along the length of a triple bond (Fig. 5). As a result, there is no restriction of rotation for groups joined by a triple bond (as compared with alkenes), and if rotation would occur, no new compound would form.
Bond Lengths of Ethyne, Ethene, and Ethane
** The carbon–carbon triple bond of ethyne is shorter than the carbon–carbon double bond of ethene, which in turn is shorter than the carbon–carbon single bond of ethane. The reason is that bond lengths are affected by the hybridization states of the carbon atoms involved.
(1) The greater the s orbital character in one or both atoms, the shorter is the bond. This is because s orbitals are spherical and have more electron density closer to the nucleus than do p orbitals.
(2) The greater the p orbital character in one or both atoms, the longer is the bond. This is because p orbitals are lobe-shaped with electron density extending away from the nucleus.
** In terms of hybrid orbitals, an sp hybrid orbital has 50% s character and 50% p character. An sp2 hybrid orbital has 33% s character and 67% p character. An sp3 hybrid orbital has 25% s character and 75% p character. The overall trend, therefore, is as follows:
(3) Bonds involving sp hybrids are shorter than those involving sp2 hybrids, which are shorter than those involving sp3 hybrids. This trend holds true for both C-C and C-H bonds.
Reference: Organic chemistry / T.W. Graham Solomons , Craig B.Fryhle , Scott A.snyder , / ( eleventh edition) / 2014.