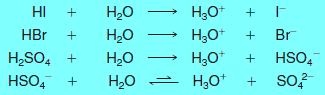Bronsted-Lowry theory for acids and bases
– In this subject, we will discuss Bronsted-Lowry theory for acids and bases
Acid-base reactions
– We begin our study of chemical reactions and mechanisms by examining some of the basic principles of acid-base chemistry.
– There are several reasons for doing this:
(1) Many of the reactions that occur in organic chemistry are either acid-base reactions themselves or they involve an acid-base reaction at some stage.
(2) Acid-base reactions are simple fundamental reactions that will enable you to see how chemists use curved arrows to represent mechanisms of reactions and how they depict the processes of bond breaking and bond making that occur as molecules react.
Bronsted-Lowry Acids and Bases
– Two classes of acid-base reactions are fundamental in organic chemistry: Bronsted–Lowry and Lewis acid-base reactions.
– We start our discussion with Bronsted–Lowry acid-base reactions.
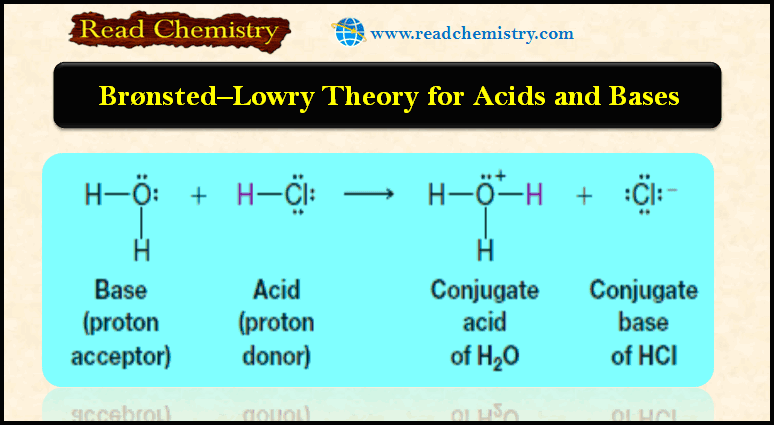
(a) Bronsted-Lowry acid-base reactions involve the transfer of protons.
(b) A Bronsted-Lowry acid is a substance that can donate (or lose) a proton.
(c) Bronsted-Lowry base is a substance that can accept (or remove) a proton.
– Let us consider some examples:
Reaction between HCl (gas) with water
– Hydrogen chloride (HCl), in its pure form, is a gas.
– When HCl gas is bubbled into water, the following reaction occurs.
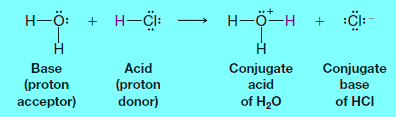
– In this reaction, hydrogen chloride donates a proton; therefore it acts as a Bronsted-Lowry acid.
– Water accepts a proton from hydrogen chloride; thus water serves as a Bronsted-Lowry base.
– The products are a hydronium ion (H3O+) and chloride ion (Cl–).
Important Notes for Bronsted-Lowry Theory
Note (1): Just as we classified the reactants as either an acid or a base, we also classify the products in a specific way:
(a) The molecule or ion that forms when an acid loses its proton is called the conjugate base of that acid.
– In the above example, the chloride ion is the conjugate base.
(b) The molecule or ion that forms when a base accepts a proton is called the conjugate acid.
– Hydronium ion is the conjugate acid of water.
Note (2): Hydrogen chloride is considered a strong acid because the transfer of its proton in water proceeds essentially to completion.
– Other strong acids that completely transfer a proton when dissolved in water are hydrogen iodide, hydrogen bromide, and sulfuric acid.
Note (3): The extent to which an acid transfers protons to a base, such as water, is a measure of its strength as an acid.
– Acid strength is therefore a measure of the percentage of ionization and not of concentration.
Note (4): Sulfuric acid is called diprotic acid because it can transfer two protons.
– Transfer of the first proton occurs completely, while the second is transferred only to the extent of about 10% (hence the equilibrium arrows in the equation for the second proton transfer).
Acids and Bases in Water
– Hydronium ion is the strongest acid that can exist in water to any significant extent.
– Any acid stronger than hydronium ion will simply transfer its proton to a water molecule to form hydronium ions.
– Hydroxide ion is the strongest base that can exist in water to any significant extent.
– Any base stronger than hydroxide will remove a proton from water to form hydroxide ions.
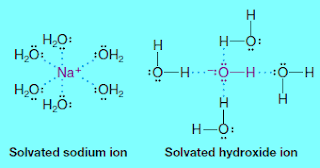
– When an ionic compound dissolves in water the ions are solvated.
– With sodium hydroxide, for example, the positive sodium ions are stabilized by interaction with unshared electron pairs of water molecules, and the hydroxide ions are stabilized by hydrogen bonding of their unshared electron pairs with the partially positive hydrogens of water molecules.
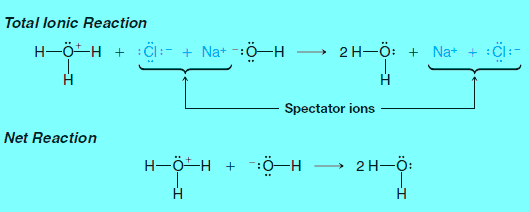
– When an aqueous solution of sodium hydroxide is mixed with an aqueous solution of hydrogen chloride (hydrochloric acid), the reaction that occurs is between hydronium and hydroxide ions.
– The sodium and chloride ions are called spectator ions because they play no part in the acid-base reaction:
– What we have just said about hydrochloric acid and aqueous sodium hydroxide is true when solutions of all aqueous strong acids and bases are mixed.
– The net ionic reaction is simply.