Atomic Number and Mass Number
– In this subject, we will discuss the Atomic Number and Mass Number
Mosley’s Determination of Atomic Number
– The discovery that an atom has a nucleus that carries a positive charge raised the question: What is the magnitude of the positive charge?
– This question was answered by Henry Mosley in 1913.
– Hitherto atomic number was designated as the ‘position number’ of a particular element in the Periodic Table.
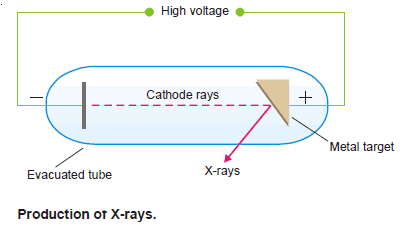
– Mosley found that when cathode rays struck different elements used as anode targets in the discharge tube, characteristic X-rays were emitted.
– The wavelength of these X-rays decreases in a regular manner in passing from one element to the next one in order in the Periodic Table.
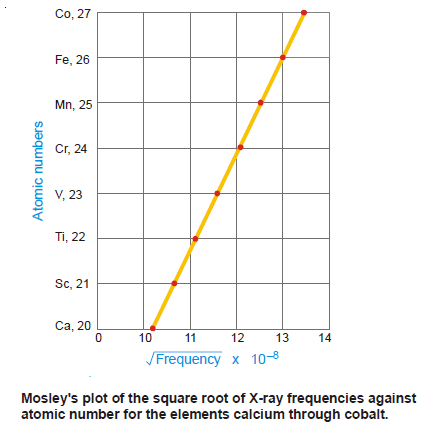
– Mosley plotted the atomic number against the square root of the frequency of the X-rays emitted and obtained a straight line which indicated that atomic number was not a mere ‘position number’ but a fundamental property of the atom.
– He further made a remarkable suggestion that the wavelength (or frequency) of the emitted X-rays was related to the number of positive charges or protons in the nucleus.
– The wavelength changed regularly as the element that came next in the Periodic Table had one proton (one unit atomic mass) more than the previous one.
– Mosley calculated the number of units of positive charge on the nuclei of several atoms and established that:
– The atomic Number of an element is equal to the number of protons in the nucleus of the atom of that element.
– Since the atom as a whole is electrically neutral, the atomic number (Z) is also equal to the number of extranuclear electrons.
– Thus hydrogen (H) which occupies the first position in the Periodic Table has atomic number 1.
– This implies that it has a nucleus containing one proton (+1) and one extranuclear electron (– 1).
– Now the term Atomic Number is often referred to as the Proton Number.
What is Mass Number?
– Mass Number, A, of the atom is The total number of protons and neutrons in the nucleus of an atom.
– In situations where it is unnecessary to differentiate between protons and neutrons, these elementary particles are collectively referred to as nucleons.
– Thus mass number of an atom is equal to the total number of nucleons in the nucleus of an atom.
– Obviously, the mass number of an atom is a whole number.
– Since electrons have practically no mass, the entire atomic mass is due to protons and neutrons, each of which has a mass almost exactly one unit.
– Therefore, the mass number of an atom can be obtained by rounding off the experimental value of atomic mass (or atomic weight) to the nearest whole number.
– For example, the atomic mass of sodium and fluorine obtained by experiment is 22.9898 and 26.9815 amu respectively.
– Thus their mass numbers are 23 for sodium and 27 for fluorine.
– Each different variety of atom, as determined by the composition of its nucleus, is called a nuclide.
Composition of The Nucleus
– Knowing the atomic number (Z) and mass number (A) of an atom, we can tell the number of protons and neutrons contained in the nucleus. By definition :
Atomic Number, Z = Number of protons
Mass Number, A = Number of protons + Number of neutrons
∴ The number of neutrons is given by the expression:
N = A – Z
Solved Problems on atomic Number and Mass Number
Uranium has atomic number 92 and atomic weight 238.029. Give the number of electrons, protons and neutrons in its atom.
Solution
– Atomic Number of uranium = 92
∴ Number of electrons = 92
and Number of protons = 92
– The number of neutrons (N) is given by the expression:
N = A – Z
– Mass Number (A) is obtained by rounding off the atomic weight:
= 238.029 = 238
∴ N = 238 – 92 = 146
– Thus uranium atom has 92 electrons, 92 protons, and 146 neutrons.
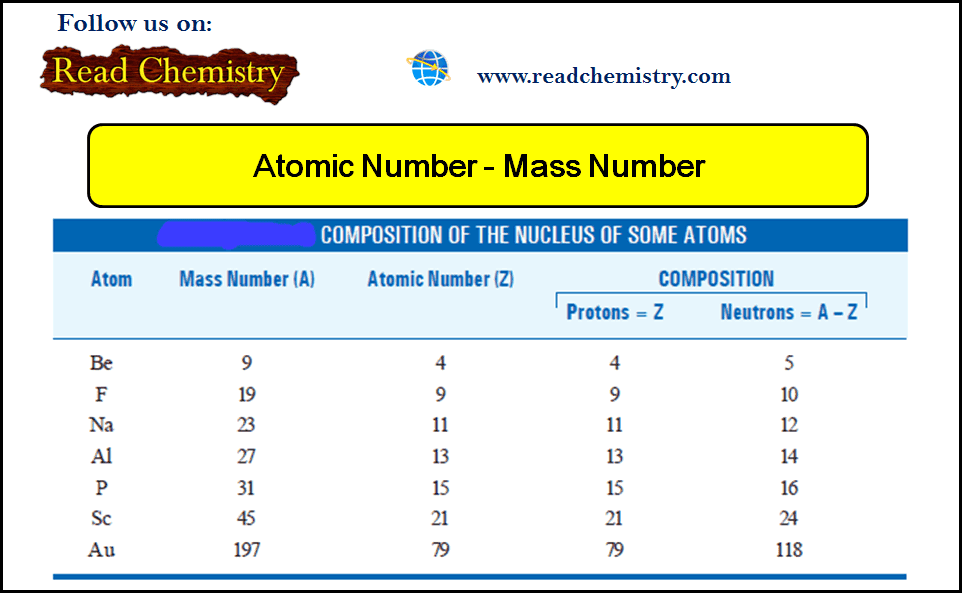
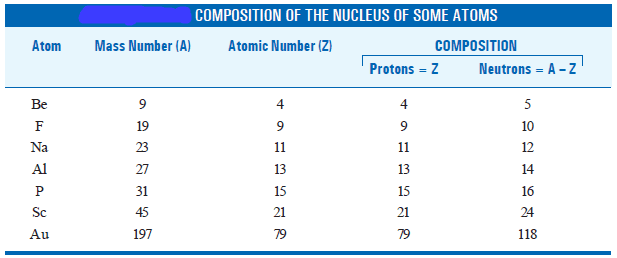
Atomic Number and Mass Number for some atoms
– The composition of nuclei of some atoms is given in the following table:
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition.