Rutherford’s Atomic Model (Experiment, Postulates, weakness)
– In this subject, we will discuss the Rutherford’s Atomic Model (Experiment, Postulates, weakness)
Alpha particles
– Alpha particles are shot out from radioactive elements with very high speed.
– For example, they come from radium atoms at a speed of 1.5 × 107m/sec.
– Rutherford identified them to be di-positive helium ions, He2+ or 4He2.
– Thus an alpha particle has 2+ charge and 4 amu mass.
– α-Particles are also formed in the discharge tube that contains helium,
– It has twice the charge of a proton and about 4 times its mass.
Conclusion
– Though α-particle is not a fundamental particle of the atom (or subatomic particle) but because of its high energy (1/2 mv2), Rutherford thought of firing them like bullets at atoms and thus obtain information about the structure of the atom.
(1) He bombarded nitrogen and other light elements by α-particles when H+ ions or protons were produced.
– This showed the presence of protons in atoms other than hydrogen atom.
(2) He got a clue to the presence of a positive nucleus in the atom as a result of the bombardment of thin foils of metals.
Rutherford’s Atomic Model – The Nuclear Atom
Having known that atom contains electrons and a positive ion, Rutherford proceeded to perform experiments to know as to how and where these were located in the atom.
Experiment of Rutherford’s Atomic Model
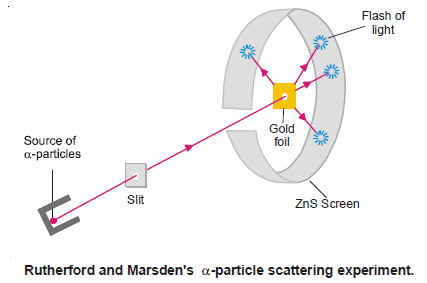
In 1909 Rutherford and Marsden performed their historic Alpha Particle-Scattering Experiment, using the apparatus illustrated in the following figure:
They directed a stream of very highly energetic α-particles from a radioactive source against a thin gold foil provided with a circular fluorescent zinc sulfide screen around it.
– Whenever an α-particle struck the screen, a tiny flash of light was produced at that point.
Observations
Rutherford and Marsden noticed that:
(1) most of the α-particles passed straight through the gold foil and thus produced a flash on the screen behind it.
– This indicated that gold atoms had a structure with plenty of empty space.
(2) To their great astonishment, tiny flashes were also seen on other portions of the screen, some time in front of the gold foil.
– This showed that gold atoms deflected or ‘scattered’ α-particles through large angles so much so that some of these bounced back to the source.
Postulates of Rutherford’s Atomic Model
– Based on these observations, Rutherford proposed a model of the atom which is named after him.
– This is also called the Nuclear Atom.
– According to it:
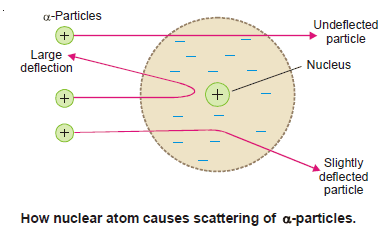
(1) The atom has a tiny dense central core or the nucleus which contains practically the entire mass of the atom, leaving the rest of the atom almost empty.
– The diameter of the nucleus is about 10-13 cm as compared to that of the atom 10–8 cm.
– If the nucleus were the size of a football, the entire atom would have a diameter of about 5 miles.
– It was this empty space around the nucleus that allowed the α-particles to pass through undeflected.
(2) The entire positive charge of the atom is located on the nucleus, while electrons were distributed in vacant space around it.
– It was due to the presence of the positive charge on the nucleus that α-particle (He2+) were repelled by it and scattered in all directions.
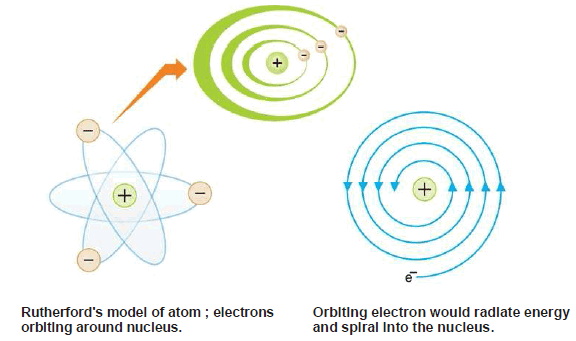
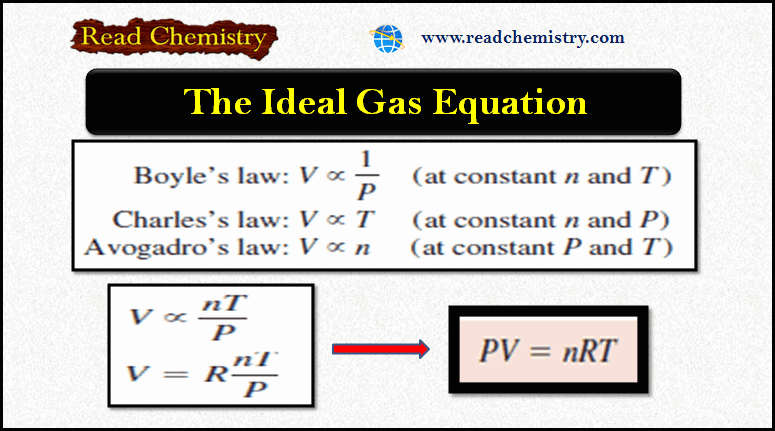
(3) The electrons were moving in orbits or closed circular paths around the nucleus like planets around the sun.
Weakness of Rutherford’s Atomic Model
– The assumption that electrons were orbiting around the nucleus was unfortunate.
– According to the classical electromagnetic theory if a charged particle accelerates around an oppositely charged particle, the former will radiate energy.
– If an electron radiates energy, its speed will decrease and it will go into spiral motion, finally falling into the nucleus.
– This does not happen actually as then the atom would be unstable which it is not.
– This was the chief weakness of Rutherford’s Atomic Model
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.