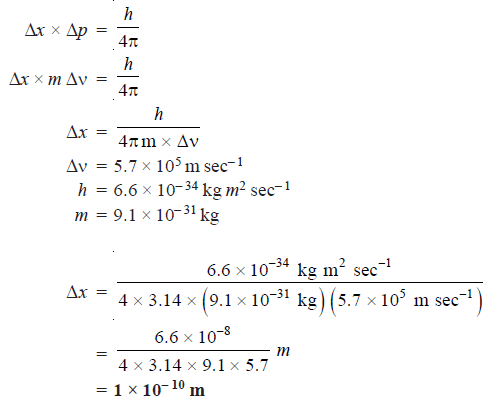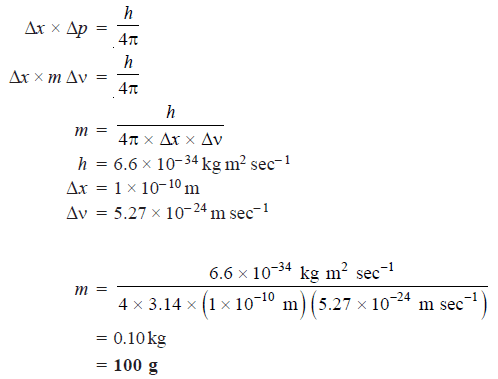Heisenberg’s uncertainty Principle
– Heisenberg’s uncertainty Principle: As it is impossible to know the position and the velocity of any one electron on account of its small size, the best we can do is to speak of the probability or relative chance of finding an electron with a probable velocity.
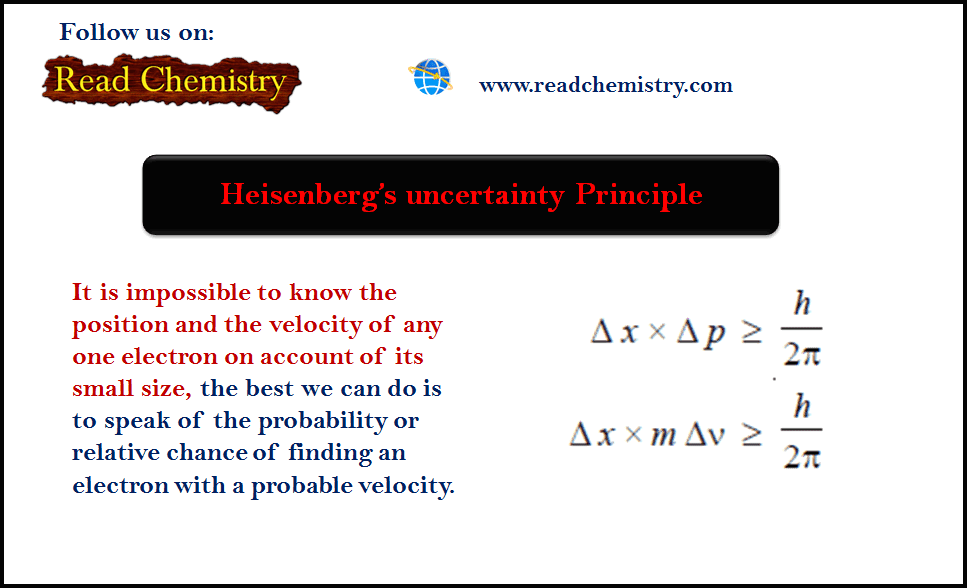
Heisenberg’s Uncertainty Principle
– One of the most important consequences of the dual nature of matter is the uncertainty principle developed by Werner Heisenberg in 1927.
– This principle is an important feature of wave mechanics and discusses the relationship between a pair of conjugate properties (those properties that are independent) of a substance.
– According to the uncertainty principle, it is impossible to know simultaneously both the conjugate properties accurately.
– For example, the position and momentum of a moving particle are interdependent and thus conjugate properties also.
– Both the position and the momentum of the particle at any instant cannot be determined with absolute exactness or certainty.
– If the momentum (or velocity) be measured very accurately, a measurement of the position of the particle correspondingly becomes less precise.
– On the other hand, if the position is determined with accuracy or precision, the momentum becomes less accurately known or uncertain.
– Thus certainty of determination of one property introduces uncertainty of determination of the other.
Heisenberg’s relationship
– The uncertainty in measurement of position, Δx, and the uncertainty of determination of momentum, Δp (or Δmv), are related by Heisenberg’s relationship as:
where (h) is Planck’s constant.
– It may be pointed out here that there exists a clear difference between the behaviour of large objects like a stone and small particles such as electrons.
– The uncertainty product is negligible in case of large objects.
– For a moving ball of iron weighing 500 g, the uncertainty expression assumes the form:
– which is very small and thus negligible. Therefore for large objects, the uncertainty of measurements is practically nil.
– But for an electron of mass m = 9.109 × 10–28 g, the product of the uncertainty of measurements is quite large as:
– This value is large enough in comparison with the size of the electron and is thus in no way negligible.
– If position is known quite accurately i.e., (Δx) is very small, the uncertainty regarding velocity (Δv) becomes immensely large and vice versa.
– It is therefore very clear that the uncertainty principle is only important in considering measurements of small particles comprising an atomic system.
Physical Concept of Uncertainty Principle
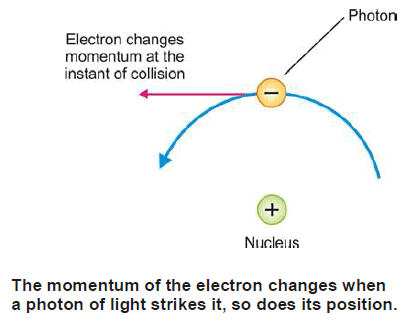
– The physical concept of uncertainty principle becomes illustrated by considering an attempt to measure the position and momentum of an electron moving in Bohr’s orbit.
– To locate the position of the electron, we should devise an instrument (supermicroscope) to see the electron.
– A substance is said to be seen only if it could reflect light or any other radiation from its surface.
– Because the size of the electron is too small, its position at any instant may be determined by a supermicroscope employing light of very small wavelength (such as X-rays or γ-rays).
– A photon of such a radiation of small (λ), has a great energy and therefore has quite large momentum.
– As one such photon strikes the electron and is reflected, it instantly changes the momentum of the electron.
– Now the momentum gets changed and becomes more uncertain as the position of the electron is being determined.
– Thus it is impossible to determine the exact position of an electron moving with a definite velocity (or possessing definite energy).
– It appears clear that Bohr’s picture of an electron as moving in an orbit with fixed velocity (or energy) is completely untenable.
– As it is impossible to know the position and the velocity of any one electron on account of its small size, the best we can do is to speak of the probability or relative chance of finding an electron with a probable velocity.
– The old classical concept of Bohr has now been discarded in favour of the probability approach.
Solved Problems on Heisenberg’s Uncertainty Principle
Problem (1): Calculate the uncertainty in the position of an electron if the uncertainty in velocity is 5.7 × 105 m sec–1.
Solution:
According to Heisenberg’s uncertainty principle:
Problem (2): The uncertainty in the position and velocity of a particle are 10–10 m and 5.27 × 10–24 m sec–1respectively. Calculate the mass of the particle.
Solution:
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition.