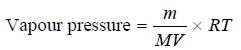Vapour Pressure , Factors affecting on Vapour Pressure
– The vapour pressure of a liquid is defined as the pressure exerted by the vapour in equilibrium with the liquid at a fixed temperature.
Vapour Pressure
– When a liquid is placed in an open vessel, it evaporates.
– The molecules in the liquid are moving with different kinetic energies.
– The molecules that possess above-average kinetic energies can overcome the intermolecular forces that hold them in the liquid.
– These energetic molecules escape from the liquid surface as vapour.
– The process by which molecules of a liquid go into the gaseous state (vapours) is called Vaporization or Evaporation.
– The reverse process whereby gas molecules become liquid molecules is called Condensation.
– If the liquid is placed in a closed vessel (Fig. 1.), the molecules with high kinetic energies escape into space above the liquid.
– As the number of molecules in the gas phase increases, some of them strike the liquid surface and are recaptured (condensation).
– A stage comes when the number of molecules escaping from the liquid is equal to the number of molecules returning to the liquid.
– In other words, the rate of evaporation exactly equals the rate of condensation.
– Thus a dynamic equilibrium is established between the liquid and the vapour at the given temperature.
Liquid ↔ Vapour
– Now the concentration of the vapour in the space above the liquid will remain unchanged with the lapse of time.
– Hence the vapor will exert a definite pressure at the equilibrium.
Definition of the vapour pressure of a liquid
– It is defined as the pressure exerted by the vapour in equilibrium with the liquid at a fixed temperature.
– The vapour pressures of various liquids differ considerably, depending upon the identity of the liquid with its particular intermolecular forces.
– Thus ethanol having weaker hydrogen bonding than water, evaporates faster than water.
– Hence we expect that ethanol will have higher vapour pressure than water at a given temperature.
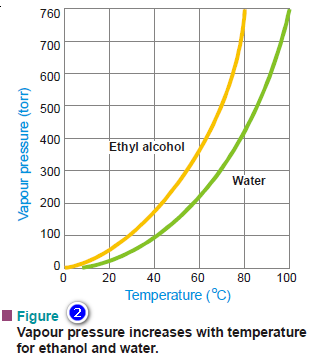
– As shown by the actual plot vapour pressure versus temperature, the vapour pressures of ethanol and water at 60ºC are about 350 torr and 150 torr respectively.
Effect of Temperature on Vapour Pressure
– If the temperature of the liquid is increased, the vapour pressure will increase.
– This is so because at higher temperatures more molecules in the liquid will have larger kinetic energy and will break away from the liquid surface.
– Therefore the concentration of vapour molecules will increase before the equilibrium is re-established.
– Also, at higher temperatures, the average kinetic energy of the vapour molecules will increase.
– Both vapour concentration and kinetic energy are proportional to temperature.
– Therefore, any increase in temperature will increase vapour pressure.
– From the experimental curves shown in Fig. 2, it is clear that for both ethyl alcohol and water, the vapour pressure rises with an increase in temperature.
Determination of Vapour Pressure
– The vapour pressure of a given liquid can be measured by Static method or Dynamic method.
(1) The Static Method
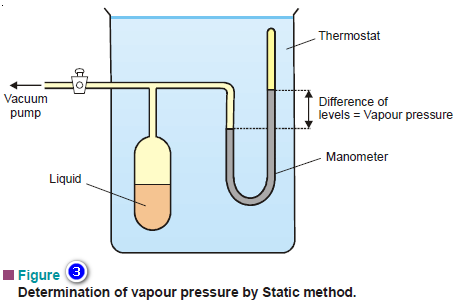
– A simplified apparatus used for the static method is shown in Fig.3.
– A sufficient amount of the liquid whose vapour pressure is to be determined is placed in the bulb connected to a mercury manometer and a vacuum pump.
– All the air from the bulb is removed by working the vacuum pump and the stopcock closed. A part of the liquid evaporates.
– The system is then maintained at a fixed temperature for enough time so that the equilibrium is established.
– The difference in the levels of mercury in the manometer is equal to the vapour pressure of the liquid.
– By adjusting the thermostat at a different temperature, the vapour pressure of the liquid at another temperature can be determined.
– This method is used for liquids having vapour pressures up to one atmosphere.
(2) The Dynamic Method
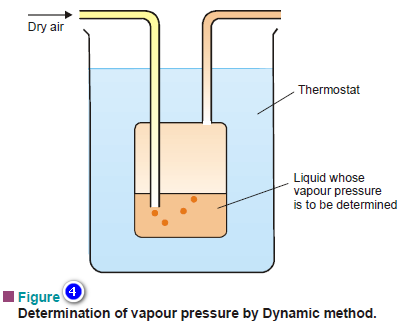
– The apparatus used for the dynamic method is illustrated in Fig 4.
– An inert gas is passed through the given liquid at a constant temperature (T).
– The gas saturated with the vapour of the liquid leaves the flask at the exit tube.
– If (V) be the volume of the gas passed and (m) the loss in weight of the liquid, the vapour pressure is given by the expression:
– where M = molecular weight of the liquid and R = gas constant.
– This method is particularly suited for liquids of very low vapour pressure.
Effect of Vapour Pressure on Boiling Points
– When a liquid is heated, tiny bubbles are formed in it. These rise to the liquid surface and burst.
– The temperature at which it happens is the boiling point of the liquid.
– Let us consider an individual bubble.
– The liquid vaporises into it and the vapour pressure in the bubble keeps it in form. However, the pressure of the atmosphere exerted on the liquid top tends to collapse the bubble.
– As the bubble goes to the surface, the vapor pressure in the bubble equals the atmospheric pressure. Thus the bubble collapses.
– The boiling point of the liquid may, therefore, be defined as the temperature at which the vapor pressure of the liquid is equal to the atmospheric pressure.
– Because the atmospheric pressure varies with altitude and other conditions, the boiling points are reported at 760 torr (1 atm).
– Therefore the normal boiling point of a liquid is the temperature at which the vapour pressure of the liquid is 760 torr or 1 atm.
– As evident from Fig. 2, the boiling point of ethanol is 78ºC and of water, 100ºC.
– The boiling point of a liquid can be lowered by reducing the external pressure by the vacuum pump.
– Then the vapor pressure of the liquid is equal to the external pressure at a lower temperature.
– The boiling point of a liquid can be increased by raising the external pressure.
– Thus the vapor pressure of the liquid is equal to the external pressure at a higher temperature.
– A domestic pressure cooker works on this principle.
– The pressure inside the cooker is maintained above one atmosphere and the liquid contained in it would boil at a higher temperature than 100ºC.
– Thus the food is cooked in a shorter time.