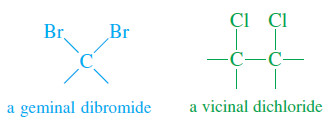Nomenclature of Alkyl Halides
Introduction to Alkyl Halides
– In this subject , we consider Nomenclature of alkyl halides.
– Our study of organic chemistry is organized into families of compounds classified by their functional groups.
– We use alkyl halides to introduce substitution and elimination, two of the most important types of reactions in organic chemistry.
– Stereochemistry will play a major role in our study of these reactions.
– Many other reactions show similarities to substitution and elimination, and the techniques introduced will be used throughout our study of organic reactions.
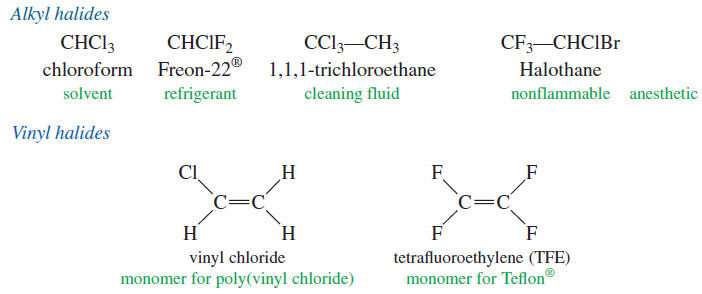
– There are three major classes of halogenated organic compounds: the alkyl halides, the vinyl halides, and the aryl halides.
– An alkyl halide simply has a halogen atom bonded to one of the sp3 hybrid carbon atoms of an alkyl group.
– A vinyl halide has a halogen atom bonded to one of the sp2 hybrid carbon atoms of an alkene.
– An aryl halide has a halogen atom bonded to one of the sp2 hybrid carbon atoms of an aromatic ring.
– The chemistry of vinyl halides and aryl halides is different from that of alkyl halides because their bonding and hybridization are different.
– The structures of some representative alkyl halides, vinyl halides, and aryl halides are shown here, with their most common names and uses.
Nomenclature of Alkyl Halides
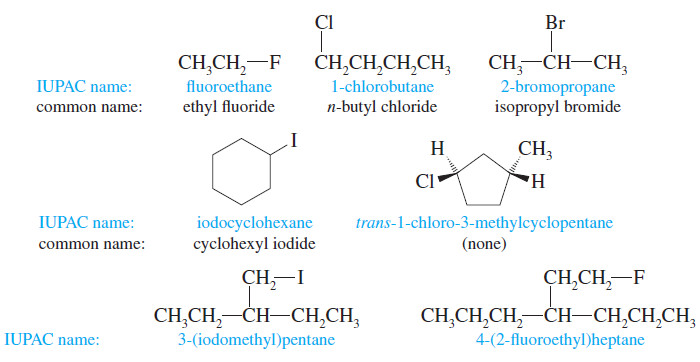
– There are two ways of naming alkyl halides.
– The systematic (IUPAC) nomenclature treats an alkyl halide as an alkane with a halo- substituent:
Fluorine is fluoro-
chlorine is chloro-
bromine is bromo-
iodine is iodo-
– The result is a systematic haloalkane name, as in 1-chlorobutane or 2-bromopropane.
– Common or “trivial” names are constructed by naming the alkyl group and then the halide, as in “isopropyl bromide.” This is the origin of the term alkyl halide.
– Common names are useful only for simple alkyl halides, such as the following:
– Some of the halomethanes have acquired common names that are not clearly related to their structures.
– A compound of formula CH2X2 (a methylene group with two halogens) is called a methylene halide; a compound of formula CHX3 is called a haloform; and a compound of formula CX4 is called a carbon tetrahalide
Classlfication of Alkyl halides
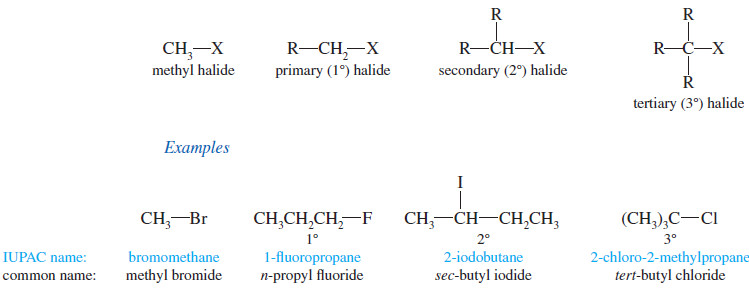
– Alkyl halides are classified according to the nature of the carbon atom bonded to the halogen.
– If the halogen-bearing carbon is bonded to one carbon atom, it is primary (1°) and the alkyl halide is a primary halide.
– If two carbon atoms are bonded to the halogen-bearing carbon, it is secondary (2°) and the compound is a secondary halide.
– A tertiary halide (3°) has three other carbon atoms bonded to the halogen bearing carbon atom.
– If the halogen-bearing carbon atom is a methyl group (bonded to no other carbon atoms), the compound is a methyl halide.
– A geminal dihalide (Latin, geminus, “twin”) has the two halogen atoms bonded to the same carbon atom.
– A vicinal dihalide (Latin, vicinus, “neighboring”) has the two halogens bonded to adjacent carbon atoms.