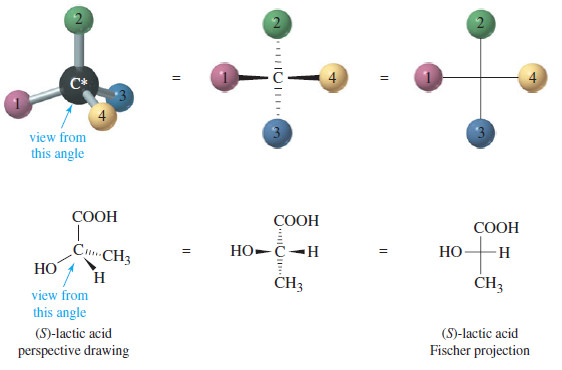
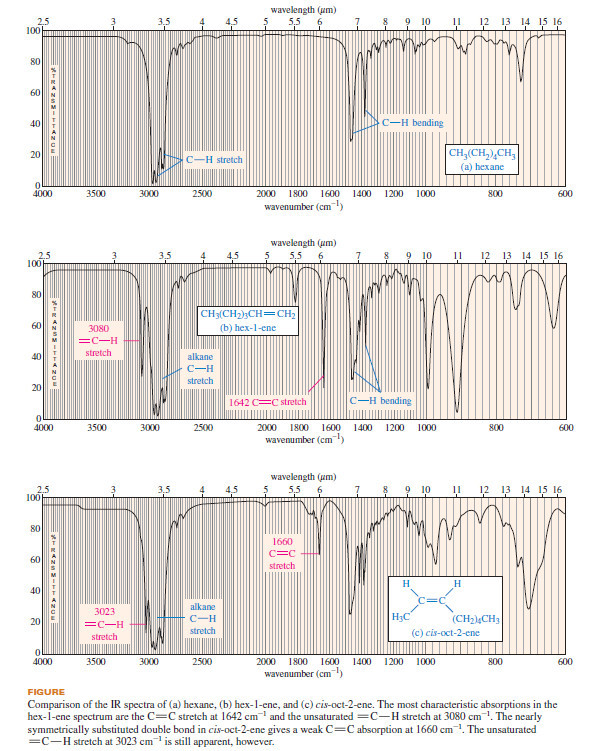
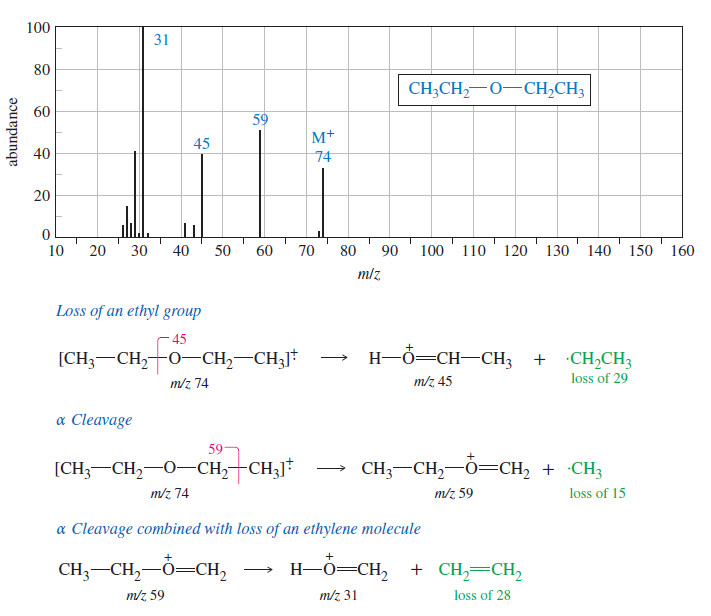
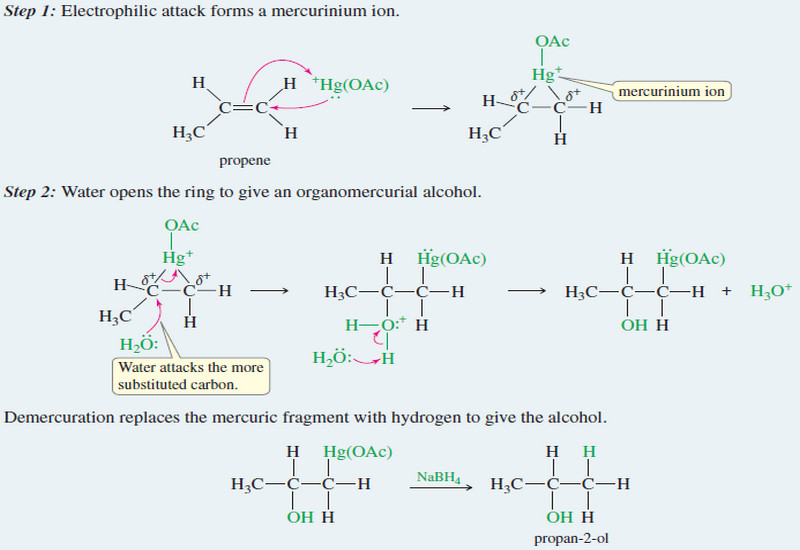
Stereochemistry of the SN2 Reaction
Stereochemistry of the SN2 Reaction
– As we have seen, the reaction SN2 requires attack by a nucleophile on the back side of an electrophilic carbon atom (see Figure)
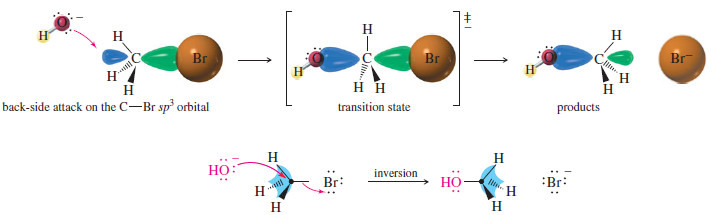
– A carbon atom can have only four filled bonding orbitals (an octet), so the leaving group must leave as the nucleophile bonds to the carbon atom.
– The nucleophile’s electrons insert into the back lobe of carbon’s sp3 hybrid orbital in its antibonding combination with the orbital of the leaving group (because the bonding MO is already filled).
– These electrons in the antibonding MO help to weaken C-Br the bond as bromide leaves.
– The transition state shows partial bonding to both the nucleophile and the leaving group.
– Back-side attack literally turns the tetrahedron of the carbon atom inside out, like an umbrella caught by the wind (the previous Figure).
– In the product, the nucleophile assumes a stereochemical position opposite the position the leaving group originally occupied.
– We call this result an inversion of configuration at the carbon atom.
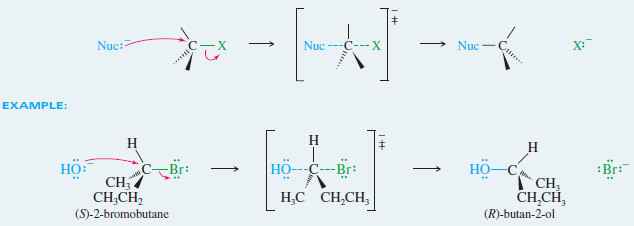
MECHANISM: Inversion of Configuration in the SN2 Reaction
Back-side attack inverts the configuration of the carbon atom
– In the case of an asymmetric carbon atom, back-side attack gives the opposite configuration of the carbon atom.
– The SN2 displacement is the most common example of a Walden inversion, a step (in a reaction sequence) where an asymmetric carbon atom undergoes inversion of configuration.
– In the 1890s, Paul Walden, of the University of T bingen (Germany), was one of the first to study reactions giving inversion of configuration.
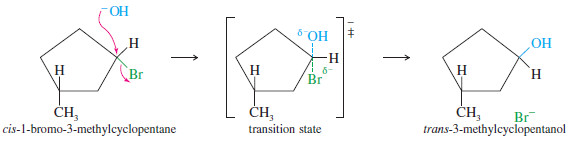
– In some cases, inversion of configuration is readily apparent.
– For example, when cis-1-bromo-3 methylcyclopentane undergoes SN2 displacement by hydroxide ion, inversion of configuration gives trans-3-methylcyclopentanol.
– The SN2 displacement is a good example of a stereospecific reaction: one in which different stereoisomers react to give different stereoisomers of the product.
– To study the mechanism of a nucleophilic substitution, we often look at the product to see if the reaction is stereospecific, with inversion of configuration.
– If it is, the mechanism is a good possibility, especially if the reaction kinetics are second order.
– In many cases (no asymmetric carbon or ring, for example), it is impossible to determine whether inversion has occurred.
– In these cases, we use kinetics and other evidence to help determine the reaction mechanism.

Thanks!