Commercially Important Alcohols
we will talk about some Commercially Important Alcohols such as: Methanol , Ethanol , isopropyl alcohol
(1) Commercially Important Alcohols: Methanol
– Methanol (methyl alcohol) was originally produced by the destructive distillation of wood chips in the absence of air. This source led to the name wood alcohol.
– During Prohibition (1919–1933), when the manufacture of alcoholic beverages was prohibited in the United States, anything called “alcohol” was often used for mixing drinks.
– Since methanol is more toxic than ethanol, this practice resulted in many cases of blindness and death.
– Today, most methanol is synthesized by a catalytic reaction of carbon monoxide with hydrogen.
– This reaction uses high temperatures and pressures and requires large, complicated industrial reactors.
– Synthesis gas, containing the hydrogen and carbon monoxide needed to make methanol, can be generated by the partial burning of coal in the presence of water.
– Careful regulation of the amount of water added allows production of synthesis gas with the correct ratio of carbon monoxide to hydrogen.
Advantages of methanol
(1) Methanol is one of the most common industrial solvents. It is cheap, relatively less toxic (compared with halogenated solvents), and it dissolves a wide variety of polar and nonpolar substances.
(2) Methanol is also a starting material for a wide variety of methyl ethers, methyl esters, and other compounds used in plastics, medicines, fuels, and solvents.
(3) Methanol is a good fuel for internal combustion engines. From 1965–2006, all the cars at the Indianapolis 500 used methanol-fueled engines. The switch from gasoline to methanol was driven by a bad fire after a crash in 1964.
(4) Methanol is less flammable than gasoline, and water is effective against methanol fires (water mixes with and dilutes methanol).
As with any alternative fuel, there are advantages and disadvantages to the use of methanol.
Disadvantages of methanol.
(1) Its high octane rating when using as fuel.
(2) low pollutant emissions
(3) lower flammability must be weighed against its lower energy content (smaller Δ H of combustion per gram), requiring 1.7 g of methanol to produce the same energy as 1 g of gasoline.
(4) Because of its excellent solvent properties, methanol is hard on rings, seals, and plastic fuel-system parts.
(5) Its tendency to burn with little or no visible flame can allow dangerous methanol fires to go undetected
(2) Commercially Important Alcohols: Ethanol
– The prehistoric discovery of ethanol probably occurred when rotten fruit was consumed and found to have an intoxicating effect.
– This discovery presumably led to the intentional fermentation of fruit juices.
– The primitive wine that resulted could be stored (in a sealed container) without danger of decomposition, and it also served as a safe, unpolluted source of water to drink.
Production of Ethanol
– Ethanol can be produced by the fermentation of sugars and starches from many different sources.
– Grains such as corn, wheat, rye, and barley are common sources, resulting in the name grain alcohol for ethanol.
– Cooking the grain, followed by addition of sprouted barley, called malt, converts some of the starches to simpler sugars.
– Brewer’s yeast is then added, and the solution is incubated while the yeast cells convert simple sugars such as glucose to ethanol and carbon dioxide.
– The alcoholic solution that results from fermentation contains only 12–15% alcohol, because yeast cells cannot survive higher concentrations.
– Distillation increases the alcohol concentration to about 40–50% (80 to 100 “proof”) for “hard” liquors.
– Distillation of ethanol–water solutions cannot increase the ethanol concentration above 95% because a solution of 95% ethanol and 5% water boils at a lower temperature (78.15 °C) than either pure water (100 °C) or pure ethanol (78.3 °C).
– Such a mixture of liquids that boils at a lower temperature than either of its components is called a minimum-boiling azeotrope.
– The 95% alcohol produced by distillation is well suited for use as a solvent and a reagent when traces of water do not affect the reaction.
– When absolute alcohol (100% ethanol) is required, the 95% azeotrope is passed through a dehydrating agent such as anhydrous calcium oxide (CaO), which removes the final 5% of water.
– Since World War II, most industrial ethanol has been synthesized directly by the catalyzed high-temperature, high-pressure, gas-phase reaction of water with ethylene. This process uses catalysts such as P2O5 tungsten oxide, or various specially treated clays
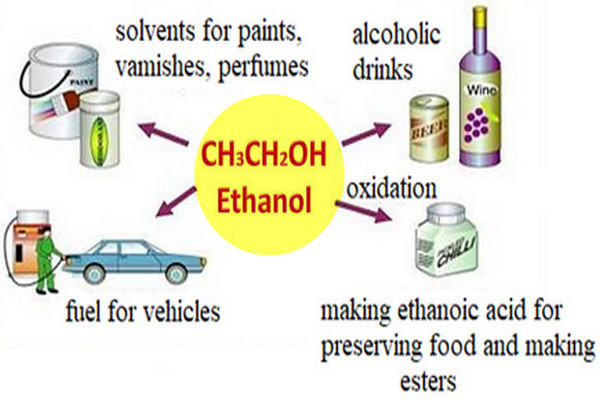
Ethanol is an excellent solvent
– Like methanol, ethanol is an excellent solvent of low toxicity that is cheap to produce.
– Unfortunately, the liquor tax makes ethanol relatively expensive.
– Use of untaxed ethanol is possible, but it requires extensive record keeping and purchase of a special license.
– Denatured alcohol is ethanol that contains impurities that make it undrinkable.
– Denatured ethanol is untaxed, but the impurities (methanol, methyl isobutyl ketone, aviation gasoline, etc.) also make it unsuitable for many laboratory uses.
Ethanol is a good motor fuel
– Like methanol, ethanol is a good motor fuel, with similar advantages and disadvantages.
– The race cars at the Indianapolis 500 have used ethanol as their primary fuel since 2006.
– A car’s carburetor must be adjusted (for a richer mixture) and fitted with alcohol-resistant seals if it is to run on pure ethanol.
– Solutions of about 10% ethanol in gasoline (gasohol) work well without any adjustments, however.
Toxicity of Ethanol
– Many people imagine ethanol to be nontoxic, and methanol to be horribly toxic.
– Actually, methanol is about twice as toxic as ethanol: Typical fatal doses for adults are about 100 mL of methanol or about 200 mL of ethanol, although smaller doses of methanol may damage the optic nerve.
– Many people die each year from underestimating ethanol’s toxicity.
– In the lab, we would never ingest even a tiny fraction of these amounts.
– Therefore, we consider these solvents to be relatively nontoxic compared with truly hazardous solvents such as benzene and chloroform.
(3) Commercially Important Alcohols: isopropyl alcohol
– Propan-2-ol (2-propanol, isopropyl alcohol) is made by the catalytic hydration of propylene.
– Isopropyl alcohol is commonly used as rubbing alcohol (rather than ethanol) because it has less of a drying effect on the skin, and it is not regulated and taxed by the government.
– Propan-2-ol is about as toxic as methanol when taken orally, but it is safer for use on the skin because it does not pass through skin as easily as methanol

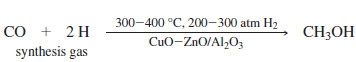


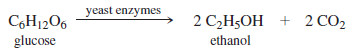

Excellent article, thanks for all the information provided