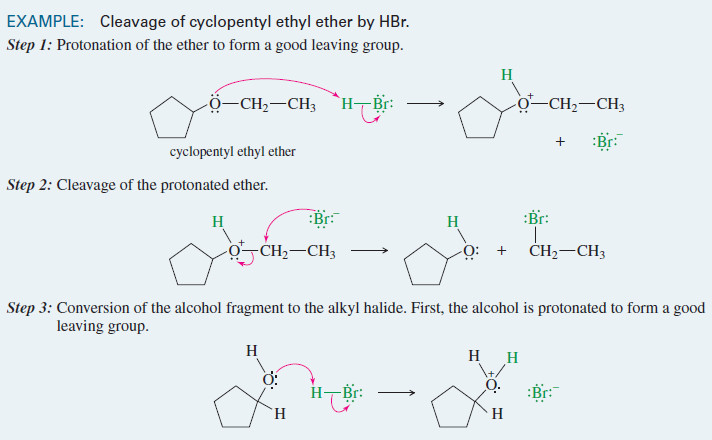
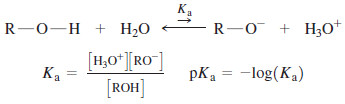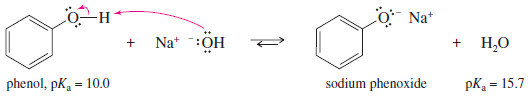Acidity of Alcohols and Phenols
Acidity of Alcohols and Phenols
– we will talk here about some Acidity of Alcohols and Phenols.
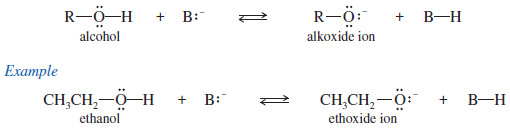
– Like the hydroxyl proton of water, the hydroxyl proton of an alcohol is weakly acidic.
– A strong base can remove the hydroxyl proton to give an alkoxide ion.
– The acidities of alcohols vary widely, from alcohols that are about as acidic as water to some that are much less acidic.
– The acid-dissociation constant, Ka , of an alcohol is defined by the equilibrium.
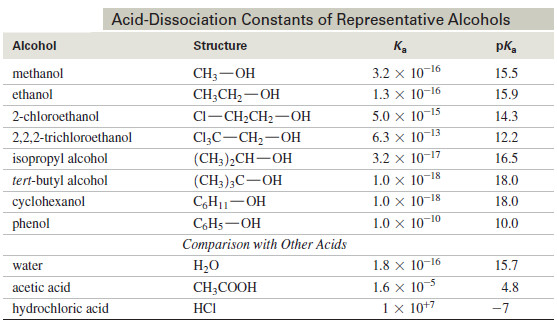
– The following Table compares the acid-dissociation constants of some alcohols with those of water and other acids
(A) Effects on Acidity of Alcohols
– The acid-dissociation constants for alcohols vary according to their structure, from about 10-16 for methanol down to about 10-18 for most tertiary alcohols.
– The acidity decreases as the substitution on the alkyl group increases, because a more highly substituted alkyl group inhibits solvation of the alkoxide ion, decreasing the stability of the alkoxide ion and driving the dissociation equilibrium toward the left.
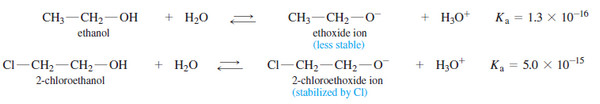
– The Table above shows that substitution by electron-withdrawing halogen atoms enhances the acidity of alcohols.
– For example, 2-chloroethanol is more acidic than ethanol because the electron-withdrawing chlorine atom helps to stabilize the 2-chloroethoxide ion.
(B) Formation of Sodium and Potassium Alkoxides
– Alkoxide ions are strong nucleophiles and strong bases, and we have already seen many of their useful reactions.
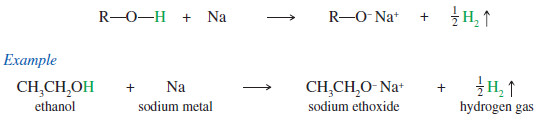
– When an alkoxide ion is needed in a synthesis, it is often formed by the reaction of sodium or potassium metal with the alcohol.
– This is an oxidation–reduction, with the metal being oxidized and the hydrogen ion reduced to form hydrogen gas.
– Hydrogen bubbles out of the solution, leaving the sodium or potassium salt of the alkoxide ion.
– The more acidic alcohols, like methanol and ethanol, react rapidly with sodium to form sodium methoxide and sodium ethoxide.
– Secondary alcohols, such as propan-2-ol, react more slowly.
– Tertiary alcohols, such as tert-butyl alcohol, react very slowly with sodium.
– Potassium is often used with secondary and tertiary alcohols because it is more reactive than sodium, and the reaction can be completed in a convenient amount of time.
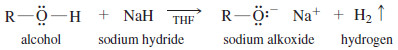
– Some alcohols react slowly with both sodium and potassium.
– In these cases, a useful alternative is sodium hydride, often in tetrahydrofuran (THF) solution.
– Sodium hydride reacts quickly to form the alkoxide, even with difficult compounds.
(C) Acidity of Phenols
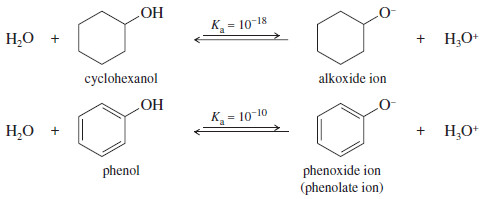
– We might expect that phenol would have about the same acidity as cyclohexanol, since their structures are similar.
– This prediction is wrong: Phenol is nearly 100 million (108) times more acidic than cyclohexanol
– Cyclohexanol is a typical secondary alcohol, with a typical acid-dissociation constant for an alcohol.
– There must be something special about phenol that makes it unusually acidic.
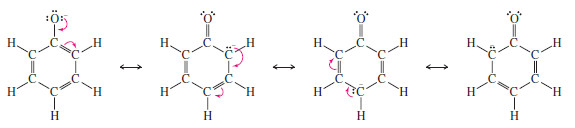
– The phenoxide ion is more stable than a typical alkoxide ion because the negative charge is not confined to the oxygen atom but is delocalized over the oxygen and three carbon atoms of the ring.
– A large part of the negative charge in the resonance hybrid still resides on the oxygen atom, since it is the most electronegative of the four atoms sharing the charge.
– But the ability to spread the negative charge over four atoms rather than concentrating it on just one atom produces a more stable ion.
– The reaction of phenol with sodium hydroxide is exothermic, and the following equilibrium lies to the right.
– Phenoxide anions are prepared simply by adding the phenol to an aqueous solution of sodium hydroxide or potassium hydroxide. There is no need to use sodium or potassium metal.
– Phenol was once called carbolic acid because of its ability to neutralize common bases.