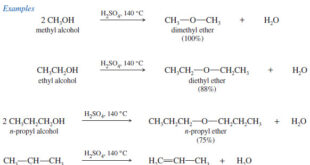
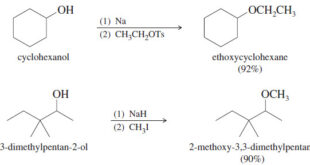
Reactions of Alcohols with Thionyl Chloride
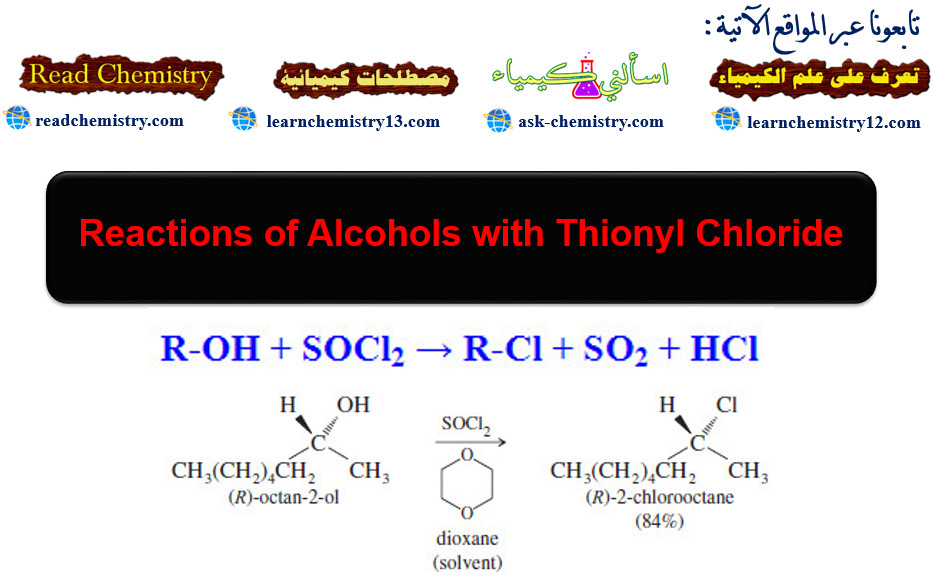
– Reactions of Alcohols with Thionyl Chloride give alkyl chloride.
– Thionyl chloride (SOCl2) is often the best reagent for converting an alcohol to an alkyl chloride.
– The by-products (gaseous SO2 and HCl) leave the reaction mixture and ensure there can be no reverse reaction.
R-OH + SOCl2 → R-Cl + SO2 + HCl
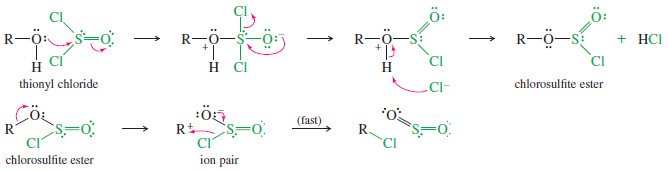
– Under the proper conditions, thionyl chloride reacts by the interesting mechanism summarized next.
– In the first step, the nonbonding electrons of the hydroxyl oxygen atom attack the electrophilic sulfur atom of thionyl chloride.
– A chloride ion is expelled, and a proton is lost to give a chlorosulfite ester.
– In the next step, the chlorosulfite ester ionizes (when R = 2° or 3°), and the sulfur atom quickly delivers chloride to the carbocation.
– When (R) is primary, chloride probably bonds to carbon at the same time that the C-O bond is breaking.
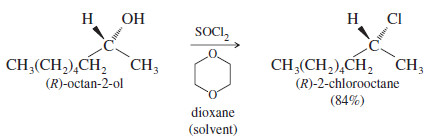
– This mechanism resembles the except that the nucleophile is delivered to the carbocation by the leaving group, usually giving retention of configuration as shown in the following example. (Under different conditions, retention of configuration might not be observed
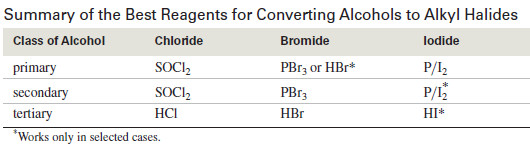
Summary of the Best Reagents for Converting Alcohols to Alkyl Halides
Problem (1)
Two products are observed in the following reaction.
(a) Suggest a mechanism to explain how these two products are formed.
(b) Your mechanism for part (a) should be different from the usual mechanism of the reaction of with alcohols.
Explain why the reaction follows a different mechanism in this case?
Problem-solving Hint
Thionyl chloride reacts with alcohols by various mechanisms that depend on the substrate, the solvent, and the temperature.
– Be cautious in predicting the structure and stereochemistry of a product unless you know the actual mechanism.
 Read Chemistry
Read Chemistry