Magnetic Shielding by Electrons
– In this topic, we will discuss the Magnetic Shielding by Electrons
Magnetic Shielding by Electrons
– Up to now, we have considered the resonance of a naked proton in a magnetic field, but real protons in organic compounds are not naked.
– They are surrounded by electrons that partially shield them from the external magnetic field.
– The electrons circulate and generate a small induced magnetic field that opposes the externally applied field.
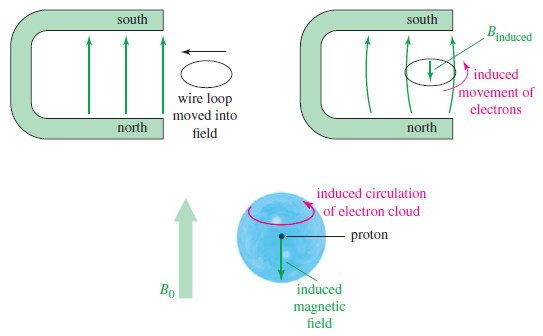
– A similar effect occurs when a loop of wire is moved into a magnetic field.
– The electrons in the wire are induced to flow around the loop in the direction shown in Figure below; this is the principle of the electric generator.
– The induced electric current creates a magnetic field that opposes the external field.
– In a molecule, the electron cloud around each nucleus acts like a loop of wire, rotating in response to the external field.
– This induced rotation is a circular current whose magnetic field opposes the external field.
– The result is that the magnetic field at the nucleus is weaker than the external field, and we say the nucleus is shielded.
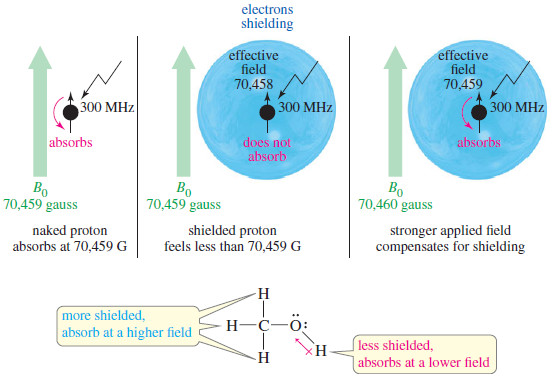
– The effective magnetic field at the shielded proton is always weaker than the external field, so the applied field must be increased for resonance to occur at a given frequency (see Figure).
Beffective = Bexternal – Bshielding
– At 300 MHz, an unshielded naked proton absorbs at 70,459 gauss, but a shielded proton requires a stronger field.
– For example, if a proton is shielded by 1 gauss when the external field is 70,459 gauss, the effective magnetic field at the proton is 70,458 gauss.
– If the external field is increased to 70,460 gauss, the effective magnetic field at the proton is increased to 70,459 gauss, which brings this proton into resonance.
– If all protons were shielded by the same amount, they would all be in resonance at the same combination of frequency and magnetic field.
– Fortunately, protons in different chemical environments are shielded by different amounts.
– In methanol, for example, the electronegative oxygen atom withdraws some electron density from around the hydroxyl proton.
– The hydroxyl proton is not shielded as much as the methyl protons, so the hydroxyl proton absorbs at a lower field than the methyl protons (but still at a higher field than a naked proton).
– We say that the hydroxyl proton is deshielded somewhat by the presence of the electronegative oxygen atom.
– Because of the diverse and complex structures of organic molecules, the shielding effects of electrons at various positions are generally different.
– A careful measurement of the field strengths required for resonance of the various protons in a molecule provides us with two important types of information:
(1) The number of different absorptions (also called signals or peaks) implies how many different types of protons are present.
(2) The amount of shielding shown by these absorptions implies the electronic structure of the molecule close to each type of proton.
– Two other aspects of the NMR spectrum we will consider are the intensities of the signals and their splitting patterns:
(3) The intensities of the signals imply how many protons of each type are present.
(4) The splitting of the signals gives information about other nearby protons.
What happens in an NMR spectrometer?
– Before discussing the design of spectrometers, let’s review what happens in an NMR spectrometer.
– Protons (in the sample compound) are placed in a magnetic field, where they align either with the field or against it.
– While still in the magnetic field, the protons are subjected to radiation of a frequency they can absorb by changing the orientation of their magnetic moment relative to the field.
– If protons were isolated, they would all absorb at the same frequency, proportional to the magnetic field.
– But protons in a molecule are partially shielded from the magnetic field, and this shielding depends on each proton’s environment.
– Thus, protons in different environments within a molecule exposed to a constant frequency absorb the radiation at different magnetic field strengths.
– The NMR spectrometer was originally developed to vary the magnetic field and plot a graph of energy absorption as a function of the magnetic field strength.
– Such a graph is called a nuclear magnetic resonance spectrum.