Emulsions : Defination, Types, Examples, Preparation
Defination of Emulsions
– Emulsions are liquid-liquid colloidal systems.
– In other words, an emulsion may be defined as a dispersion of finely divided liquid droplets in another liquid.
– Generally one of the two liquids is water and the other, which is immiscible with water, is designated as oil.
– Either liquid can constitute the dispersed phase.
Types of Emulsions
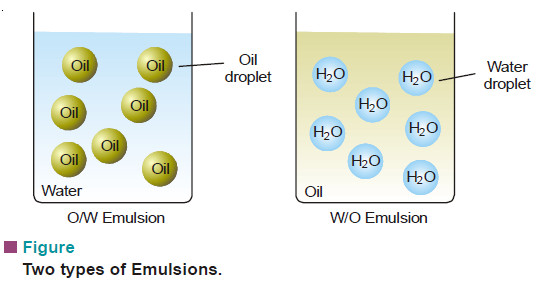
– There are two types of emulsions:
(a) Oil-in-Water type (O/W type)
(b) Water-in-Oil type (W/O type)
Examples of Emulsions
(1) Milk is an emulsion of O/W type.
– Tiny droplets of liquid fat are dispersed in water.
(2) Stiff greases are emulsions of W/O type
– water being dispersed in lubricating oil.
Preparation of Emulsions
– The dispersal of a liquid in the form of an emulsion is called emulsification.
– This can be done by agitating a small proportion of one liquid with the bulk of the other.
– It is better accomplished by passing a mixture of the two liquid through a colloid mill known as homogenizer.
– The emulsions obtained simply by shaking the two liquids are unstable.
– The droplets of the dispersed phase coalesce and form a separate layer.
– To have a stable emulsion, small amount of a third substance called the Emulsifier or Emulsifying agent is added during the preparation.
– This is usually a soap, synthetic detergent, or a hydrophilic colloid.
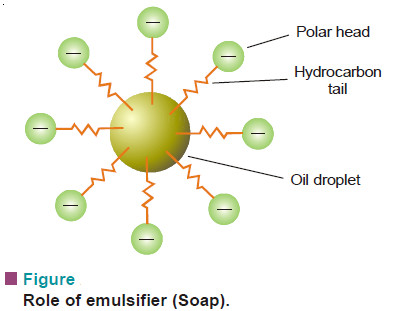
Role of Emulsifier
– The emulsifier concentrates at the interface and reduces surface tension on the side of one liquid which rolls into droplets.
– Soap, for example, is made of a long hydrocarbon tail (oil soluble) with a polar head —COO–Na+ (water soluble).
– In O/W type emulsion the tail is pegged into the oil droplet, while the head extends into water.
– Thus the soap acts as go-between and the emulsified droplets are not allowed to coalesce.
Properties of Emulsions
(1) Demulsification
– Emulsions can be broken or ‘demulsified’ to get the constituent liquids by heating, freezing, centrifuging, or by addition of appreciable amounts of electrolytes.
– They are also broken by destroying the emulsifying agent.
– For example, an oil-water emulsion stabilized by soap is broken by addition of a strong acid. The acid converts soap into insoluble free fatty acids.
(2) Dilution
– Emulsions can be diluted with any amount of the dispersion medium.
– On the other hand the dispersed liquid when mixed with it will at once form a separate layer.
– This property of emulsions is used to detect the type of a given emulsion.