Solids, liquids, and gases.
What’s the difference between Solids, liquids, and gases?
– It is easy to tell the difference between solids, liquids and gases
– A solid has a fixed shape and a fixed volume. It does not flow. Think of all the solid things around you: their shapes and volumes do not change.
– A liquid flows easily. It has a fixed volume, but its shape changes. It takes the shape of the container you pour it into.
– A gas does not have a fixed volume or shape. It spreads out to fill its container. It is much lighter than the same volume of solid or liquid.
– The following figure shows a comparison of some properties of the states of matter.
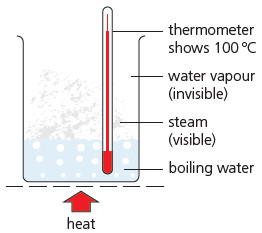
Water: solid, liquid and gas
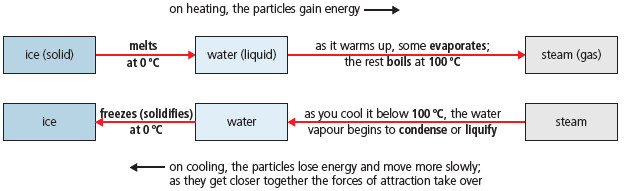
– Water can be a solid (ice), a liquid (water), and a gas (water vapour or steam). Its state can be changed by heating or cooling:
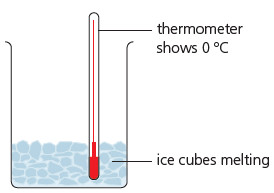
(1) Ice slowly changes to water, when it is put in a warm place. This change is called melting. The thermometer shows 0 °C until all the ice has melted. So 0 °C is called its melting point.
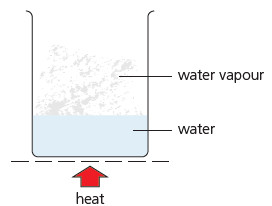
(2) When the water is heated its temperature rises, and some of it changes to water vapour. This change is called evaporation. The hotter the water gets, the more quickly it evaporates.
(3) Soon bubbles appear in the water. It is boiling. The water vapour shows up as steam. The thermometer stays at 100 °C while the water boils off. 100 °C is the boiling point of water.
And when steam is cooled, the opposite changes take place:
You can see that:
- condensing is the opposite of evaporating
- freezing is the opposite of melting
- the freezing point of water is the same as the melting point of ice, 0 °C.
Other things can change state too
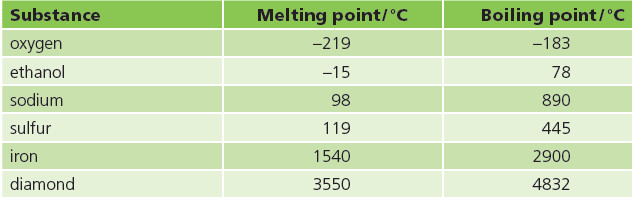
– It’s not just water! Nearly all substances can exist as solid, liquid and gas.
– Even iron and diamond can melt and boil! Some melting and boiling points are given below. Look how different they are.
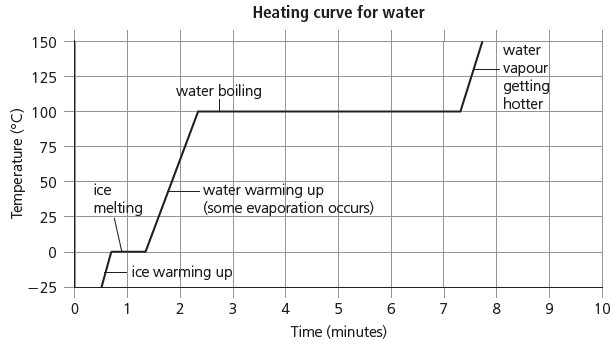
Showing changes of state on a graph
– Look at this graph. It shows how the temperature changes as a block of ice is steadily heated.
– First the ice melts to water.
– Then the water gets warmer and warmer, and eventually turns to steam:
– A graph like this is called a heating curve.
– Look at the step where the ice is melting.
– Once melting starts, the temperature stays at 0 °C until all the ice has melted.
– When the water starts to boil, the temperature stays at 100 °C until all the water has turned to steam.
– So the melting and boiling points are clear and sharp.
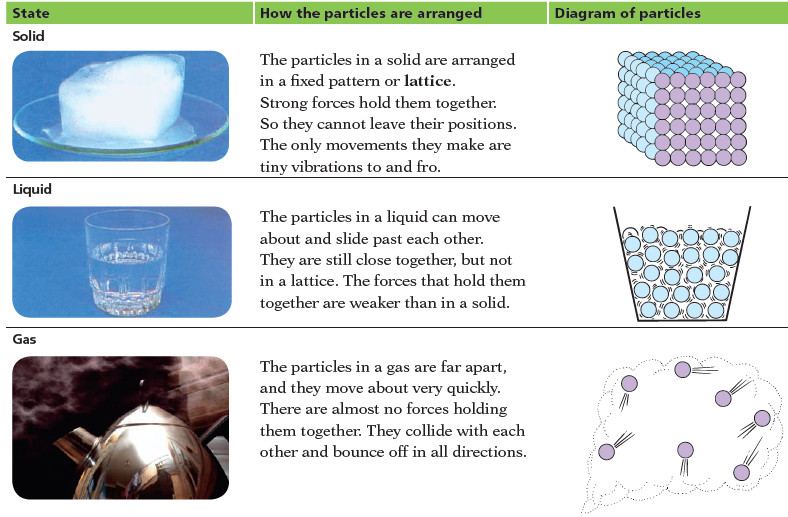
How the particles are arranged in solids, liquids, and gases?
In Solids
– The particles in a solid are arranged in a fixed pattern or lattice.
– Strong forces hold them together.
– So they cannot leave their positions. The only movements they make are tiny vibrations to and fro.
In liquids
– The particles in a liquid can move about and slide past each other.
– They are still close together, but not in a lattice.
– The forces that hold them together are weaker than in solids
In Gases
– The particles in a gas are far apart, and they move about very quickly.
– There are almost no forces holding them together.
– They collide with each other and bounce off in all directions.
Changing state
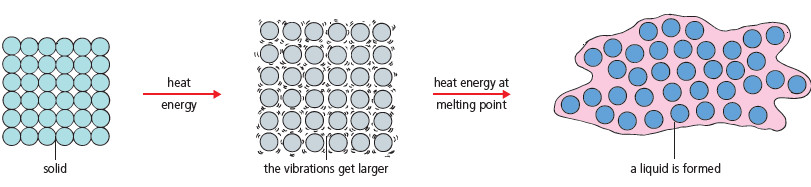
(1) Melting
– When a solid is heated, its particles get more energy and vibrate more. This makes the solid expand.
– At the melting point, the particles vibrate so much that they break away from their positions.
– The solid turns liquid.
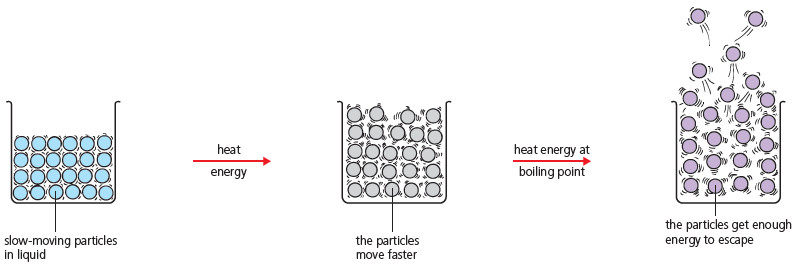
(2) Boiling
– When a liquid is heated, its particles get more energy and move faster.
– They bump into each other more often, and bounce further apart. This makes the liquid expand.
– At the boiling point, the particles get enough energy to overcome the forces between them.
– They break away to form a gas:
(3) Evaporating
– Some particles in a liquid have more energy than others.
– Even well below the boiling point, some have enough energy to escape and form a gas. This is called evaporation.
– It is why puddles of rain dry up in the sun
How much heat is needed?
– The amount of heat needed to melt or boil a substance is different for every substance.
– That’s because the particles in each substance are different, with different forces between them.
– The stronger the forces, the more heat energy is needed to overcome them.
– So the higher the melting and boiling points will be.
Reversing the changes between solids, liquids, and gases
– You can reverse those changes again by cooling.
– As a gas cools, its particles lose energy and move more slowly.
– When they collide, they do not have enough energy to bounce away.
– So they stay close, and form a liquid. On further cooling, the liquid turns to a solid.
– Look at this diagram for water:
References:
- Principles of Inorganic Chemistry / Brian W. Pfennig / 1st ed, 2015 /John Wiley & Sons, Inc/ USA.
- Inorganic Chemistry /Peter Atkins, Tina Overton, Jonathan Rourkel, Mark Weller, Fraser Armstrong, Mike Hagerman / 6th ed, 2014 /W. H. Freeman and Company/ New York, USA.
-