Nomenclature of Ethers : Rules, IUPAC Name, Common Name
Nomenclature of Ethers
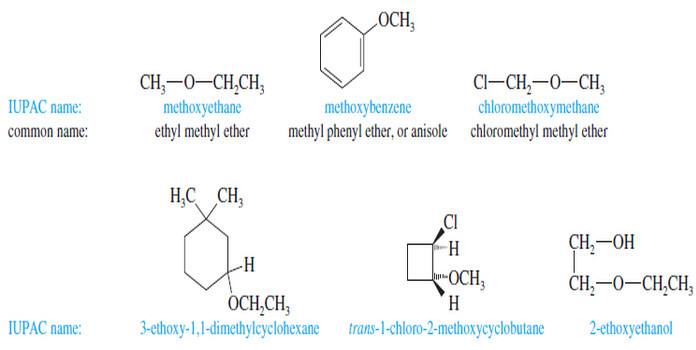
– We have been using the common nomenclature of ethers, which is sometimes called the alkyl alkyl ether system.
– The IUPAC system, generally used with more complicated ethers, is sometimes called the alkoxy alkane system.
– Common names are almost always used for simple ethers.
Common Names (Alkyl Alkyl Ether Names)
– Common names of ethers are formed by naming the two alkyl groups on oxygen and
adding the word ether.
– Under the current system, the alkyl groups should be named in alphabetical order, but many people still use the old system, which named the groups in order of increasing complexity.
– For example, if one of the alkyl groups is methyl and the other is tert-butyl, the current common name should be “tert-butyl methyl ether,” but most chemists use the older common name, “methyl tert-butyl ether” (or MTBE).
– If both groups are methyl, the name is “dimethyl ether.”
– If just one alkyl group is described in the name, it implies the ether is symmetrical, as in “ethyl ether.”
IUPAC Names (Alkoxy Alkane Names)
– IUPAC names use the more complex alkyl group as the root name, and the rest of the ether as an alkoxy group.
– For example, cyclohexyl methyl ether is named methoxycyclohexane.
– This systematic nomenclature is often the only clear way to name complex ethers.
Nomenclature of Cyclic Ethers
– Cyclic ethers are our first examples of heterocyclic compounds, containing a ring in which a ring atom is an element other than carbon.
– This atom, called the heteroatom, is numbered 1 in numbering the ring atoms.
– Heterocyclic ethers are especially important and useful ethers.
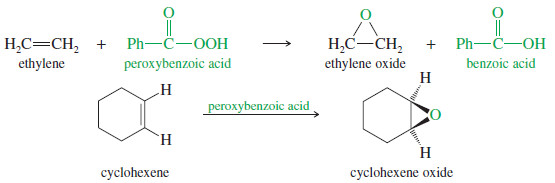
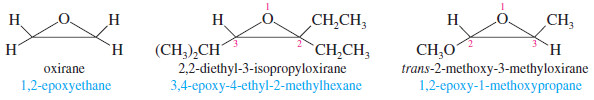
Epoxides (Oxiranes)
– Epoxides are three-membered cyclic ethers, usually formed by peroxyacid oxidation of the corresponding alkenes.
– The common name of an epoxide is formed by adding “oxide” to the name of the alkene that is oxidized.
– The following reactions show the synthesis and common names of two simple epoxides.
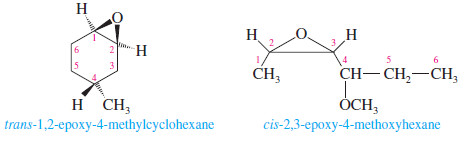
– One systematic method for naming epoxides is to name the rest of the molecule and use the term “epoxy” as a substituent, giving the numbers of the two carbon atoms bonded to the epoxide oxygen.
– Another systematic method names epoxides as derivatives of the parent compound, ethylene oxide, using “oxirane” as the systematic name for ethylene oxide.
– In this system, the ring atoms of a heterocyclic compound are numbered starting with the heteroatom and going in the direction to give the lowest substituent numbers.
– The “epoxy” system names are also listed (in blue) for comparison.
– Note that the numbering is different for the “epoxy” system names, which number the longest chain rather than the ring.
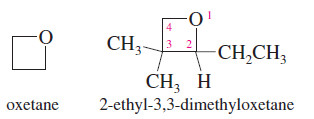
Oxetanes
– The least common cyclic ethers are the four-membered oxetanes.
– Because these four-membered rings are strained, they are more reactive than larger cyclic ethers and open-chain ethers.
– They are not as reactive as the highly strained oxiranes (epoxides), however.
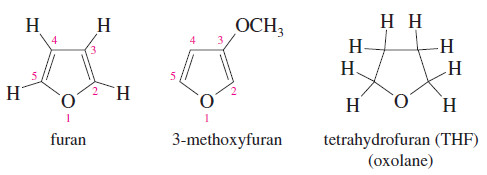
Furans (Oxolanes)
– The five-membered cyclic ethers are commonly named after an aromatic member of this group, furan.
– We consider the aromaticity of furan and other heterocycles in previous topics.
– The systematic term oxolane is also used for a five-membered ring containing an oxygen atom.
– The saturated five-membered cyclic ether resembles furan but has four additional hydrogen
atoms. Therefore, it is called tetrahydrofuran (THF).
– One of the most polar ethers, tetrahydrofuran is an excellent nonhydroxylic organic solvent for polar reagents.
– Grignard reactions sometimes succeed in THF even when they fail in diethyl ether.
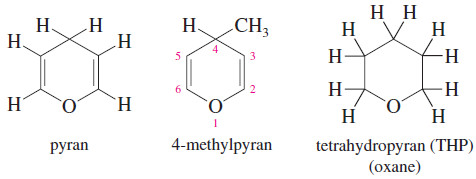
Pyrans (Oxanes)
– The six-membered cyclic ethers are commonly named as derivatives of pyran, an unsaturated ether.
– The saturated compound has four more hydrogen atoms, so it is called tetrahydropyran (THP).
– The systematic term oxane is also used for a six-membered ring containing an oxygen atom.
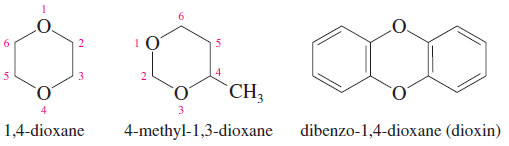
Dioxanes
– Heterocyclic ethers with two oxygen atoms in a six-membered ring are called dioxanes.
– The most common form of dioxane is the one with the two oxygen atoms in a 1,4-relationship. 1,4-Dioxane is miscible with water, and it is widely used as a polar solvent for organic reactions.
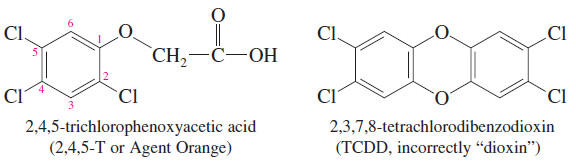
– Dioxin is a common name for dibenzo-1,4-dioxane, which is 1,4-dioxane fused with two benzene rings.
– The name “dioxin” is often used incorrectly in the news media for 2,3,7,8 tetrachlorodibenzodioxin (TCDD), a toxic contaminant in the synthesis of the herbicide called 2,4,5-T or Agent Orange.
– Surprisingly, TCDD has been in the environment for many millions of years because it is also formed in forest fires.
– Most dioxins are toxic and carcinogenic (cause cancer) because they associate with DNA and cause a misreading of the genetic code.
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.