Autoxidation of Ethers
– In this topic, we will discuss The autoxidation of Ethers.
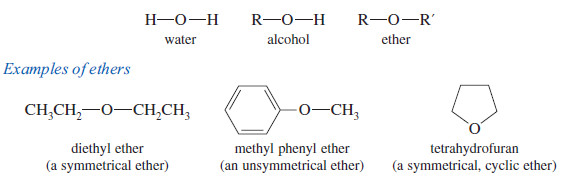
What are Ethers?
Ethers are compounds of formula R-O-R, , where R , and R, may be alkyl groups or aryl (benzene ring) groups.
– Like alcohols, ethers are related to water, with alkyl groups replacing the hydrogen atoms.
– In an alcohol, one hydrogen atom of water is replaced by an alkyl group. In an ether, both hydrogens are replaced by alkyl groups.
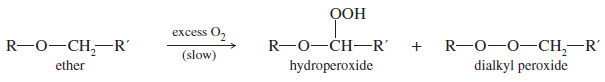
– The two alkyl groups are the same in a symmetrical ether and different in an unsymmetrical ether.
– Ethers (other than epoxides) are relatively unreactive, however, and they are not frequently used as synthetic intermediates.
– Because they are stable with many types of reagents, ethers are commonly used as solvents for organic reactions.
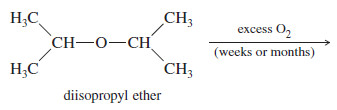
Autoxidation of Ethers
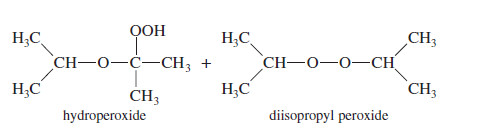
– When ethers are stored in the presence of atmospheric oxygen, they slowly oxidize to produce hydroperoxides and dialkyl peroxides, both of which are explosive. Such a spontaneous oxidation by atmospheric oxygen is called autoxidation.
Example on Autoxidation of Ethers
– Organic chemists often buy large containers of ethers and use small quantities over several months.
– Once a container has been opened, it contains atmospheric oxygen, and the autoxidation process begins.
– After several months, a large amount of peroxide may be present.
– Distillation or evaporation concentrates the peroxides, and an explosion may occur.
– Such an explosion may be avoided by taking a few simple precautions.
– Ethers should be bought in small quantities, kept in tightly sealed containers, and used promptly.
– Any procedure requiring evaporation or distillation should use only peroxide-free ether.
– Any ether that might be contaminated with peroxides should be discarded or treated to destroy the peroxides.
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.