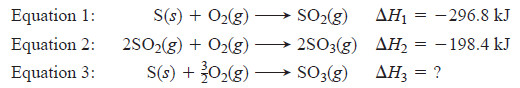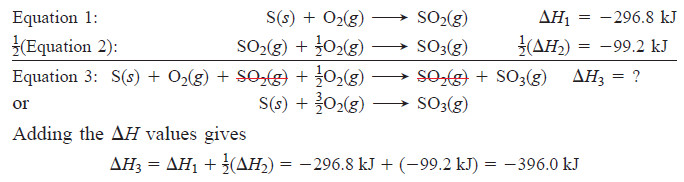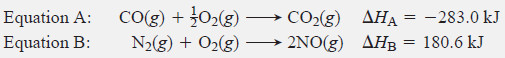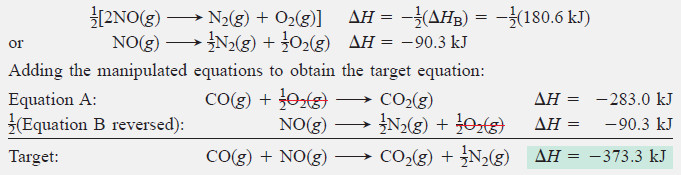Hess’s Law of Heat Summation
In this subject, we will discuss Hess’s Law of Heat Summation
Hess’s Law of Heat Summation
– Many reactions are difficult, even impossible, to carry out separately.
– A reaction may be part of a complex biochemical process; or it may take place only under extreme environmental conditions; or it may require a change in conditions while it is occurring.
– Even if we can’t run a reaction in the lab, it is still possible to find its enthalpy change.
– One of the most powerful applications of the state-function property of enthalpy (H) allows us to find the ΔH of any reaction for which we can write an equation.
– This application is based on Hess’s law of heat summation: the enthalpy change of an overall process is the sum of the enthalpy changes of its individual steps.
– To use Hess’s law, we imagine an overall reaction as the sum of a series of reaction steps, whether or not it really occurs that way.
– Each step is chosen because its ΔH is known.
– Because the overall ΔH depends only on the initial and final states, Hess’s law says that we add together the known ΔH values for the steps to get the unknown ΔH of the overall reaction.
– Similarly, if we know the ΔH values for the overall reaction and all but one of the steps, we can find the unknown ΔH of that step.
– Let’s see how we apply Hess’s law in the case of the oxidation of sulfur to sulfur trioxide, the central process in the industrial production of sulfuric acid and in the formation of acid rain.
(To introduce the approach, we’ll simplify the equations by using S as the formula for sulfur, rather than the more correct S8.)
– When we burn S in an excess of O2, sulfur dioxide (SO2) forms, not sulfur trioxide (SO3).
– Equation 1 shows this step and its ΔH.
– If we change conditions and then add more O2, we can oxidize SO2 to SO3 (Equation 2).
– In other words, we cannot put S and O2 in a calorimeter and find ΔH for the overall reaction of S to SO3 (Equation 3). But, we can find it with Hess’s law.
– The three equations are:
– Hess’s law tells us that if we manipulate Equations 1 and/or 2 so that they add up to Equation 3, then ΔH3 is the sum of the manipulated ΔH values of Equations 1 and 2.
– First, we identify Equation 3 as our “target” equation, the one whose ΔH we want to find, and we carefully note the number of moles of each reactant and product in it.
– We also note that ΔH1 and ΔH2 are the values for Equations 1 and 2 as written.
– Now we manipulate Equations 1 and/or 2 as follows to make them add up to Equation 3:
- Equations 1 and 3 contain the same amount of S, so we leave Equation 1 unchanged.
- Equation 2 has twice as much SO3 as Equation 3, so we multiply it by , being sure to halve ΔH2 as well.
- With the targeted amounts of reactants and products now present, we add Equation 1 to the halved Equation 2 and cancel terms that appear on both sides:
– Once again, the key point is that H is a state function, so the overall ΔH depends on the difference between the initial and final enthalpies only.
– Hess’s law tells us that the difference between the enthalpies of the reactants (1 mol of S and mol of O2) and that of the product (1 mol of SO3) is the same, whether S is oxidized directly to SO3 (impossible) or through the formation of SO2 (actual).
Calculating an unknown ΔH
To summarize, calculating an unknown ΔH involves three steps:
(1) Identify the target equation, the step whose ΔH is unknown, and note the number of moles of each reactant and product.
(2) Manipulate the equations with known ΔH values so that the target numbers of moles of reactants and products are on the correct sides. Remember to:
• Change the sign of ΔH when you reverse an equation.
• Multiply numbers of moles and ΔH by the same factor.
(3) Add the manipulated equations to obtain the target equation.
– All substances except those in the target equation must cancel.
– Add their ΔH values to obtain the unknown ΔH.
Solved problem
– Using Hess’s Law to Calculate an Unknown ΔH
Two gaseous pollutants that form in auto exhaust are CO and NO. An environmental chemist is studying ways to convert them to less harmful gases through the following equation:
Given the following information, calculate the unknown ΔH:
Solution:
– We note the numbers of moles of each substance in the target equation, manipulate Equations A and/or B and their ΔH values, and then add them together to obtain the target equation and the unknown ΔH.
– Noting moles of substances in the target equation: There are 1 mol each of reactants CO and NO, 1 mol of product CO2, and mol of product N2.
Manipulating the given equations:
Equation A has the same number of moles of CO and CO2 as the target, so we leave it as written.
Equation B has twice the needed amounts of N2 and NO, and they are on the opposite sides from the target; therefore, we reverse Equation B, change the sign of ΔHB, and multiply both by :
– Obtaining the desired target equation is its own check.
– Be sure to remember to change the sign of ΔH for any equation you reverse.
Summary of Hess’s Law of Heat Summation
– Because H is a state function,
ΔH = Hfinal – Hinitial
and does not depend on how the reaction takes place.
– Using Hess’s law:
ΔHtotal = ΔH1 + ΔH2 + . . . + ΔHn
we can determine ΔH of any equation by manipulating the coefficients of other appropriate equations and their known ΔH values.
References
- Principles of Inorganic Chemistry / Brian W. Pfennig / 1st ed, 2015 /John Wiley & Sons, Inc/ USA.
- Principles of general Chemistry / Martin S. Silberberg / 2nd ed, 2010 /The McGraw-Hill Companies, Inc./ USA.
- Inorganic Chemistry /Peter Atkins, Tina Overton, Jonathan Rourkel, Mark Weller, Fraser Armstrong, Mike Hagerman / 6th ed, 2014 /W. H. Freeman and Company/ New York, USA.
-