-
General Chemistry
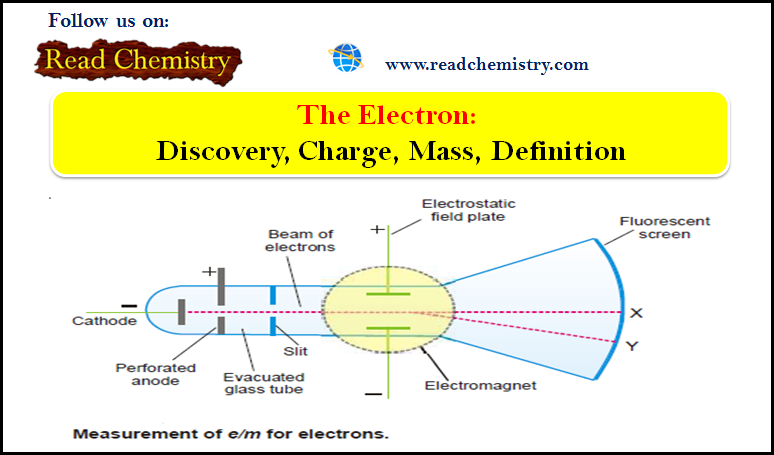
Electron: Discovery, Charge, Mass, Definition
Cathode Rays – The discovery of electron – The knowledge about the electron was derived as a result of the…
Read More » -
Organic Chemistry
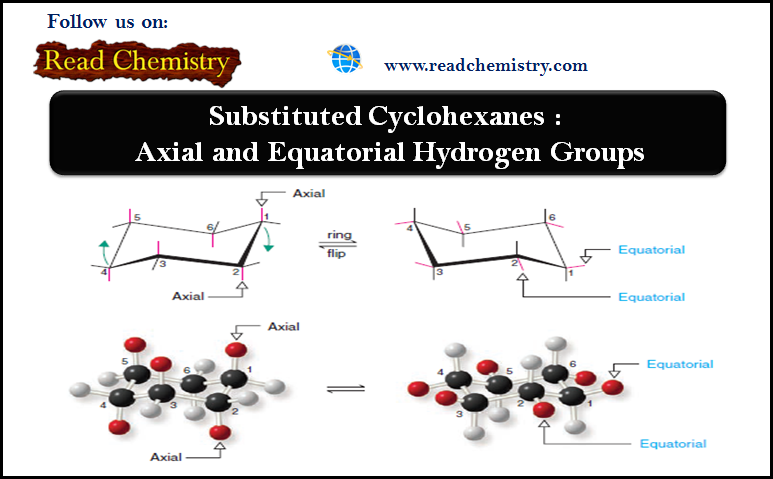
Cyclohexane: Axial and Equatorial Bonds in Cyclohexane
– In this subject, we will discuss the Substituted Cyclohexane: Axial and Equatorial Hydrogen Groups Substituted Cyclohexane: Axial and Equatorial…
Read More » -
Organic Chemistry
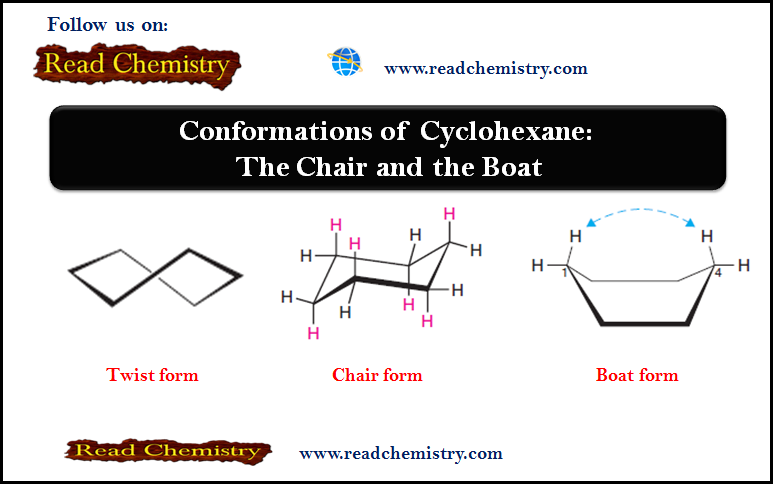
Conformations of Cyclohexane: The Chair and the Boat
– In this subject, we will discuss Conformations of Cyclohexane: The Chair and the Boat Conformations of Cyclohexane: The Chair…
Read More » -
Organic Chemistry
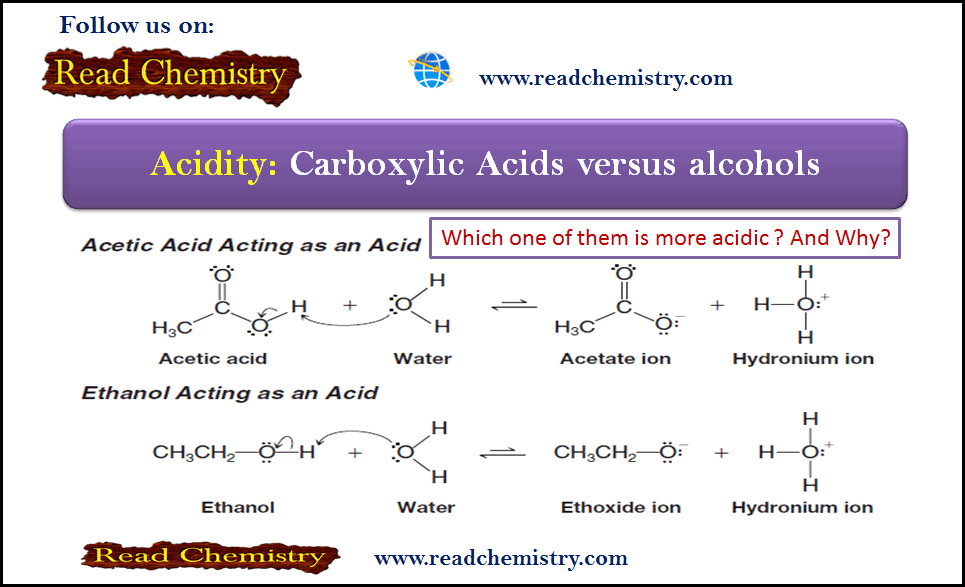
Acidity of Carboxylic Acids and Alcohols
– In this subject, we will discuss the Acidity Differences between Alcohols and Carboxylic Acids Acidity of Carboxylic Acids and…
Read More » -
General Chemistry
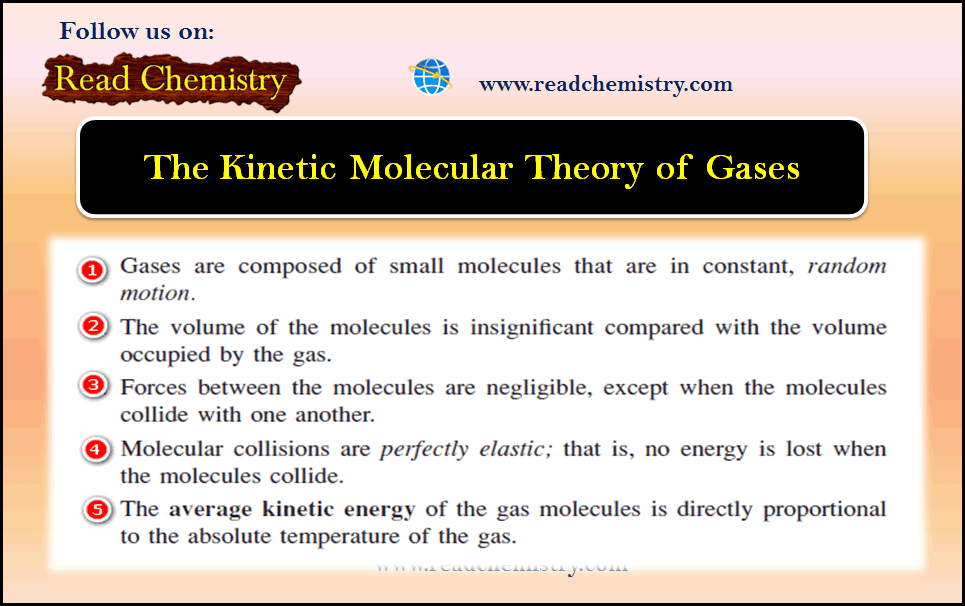
The Kinetic Molecular Theory of Gases
– In this subject, we will discuss the Kinetic Molecular Theory of Gases. Introduction to The Kinetic Molecular Theory –…
Read More » -
Analytical Chemistry
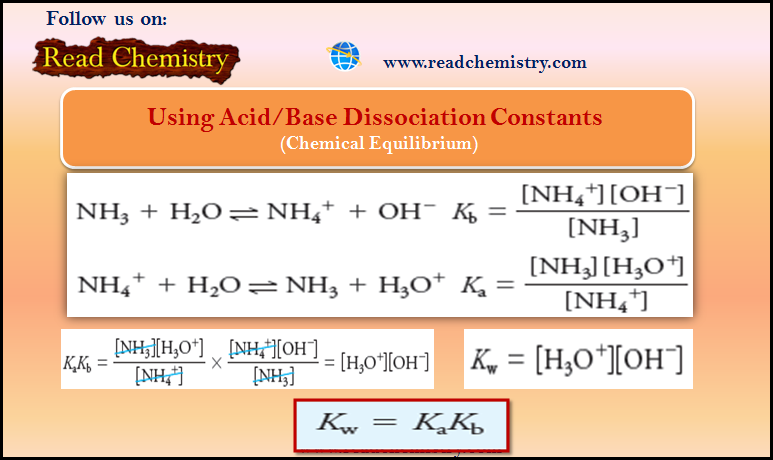
Acid and Base Dissociation Constants (Ka and Kb)
– In this subject, we will discuss the Acid and Base Dissociation Constants (Ka and Kb) Acid and Base Dissociation…
Read More » -
General Chemistry
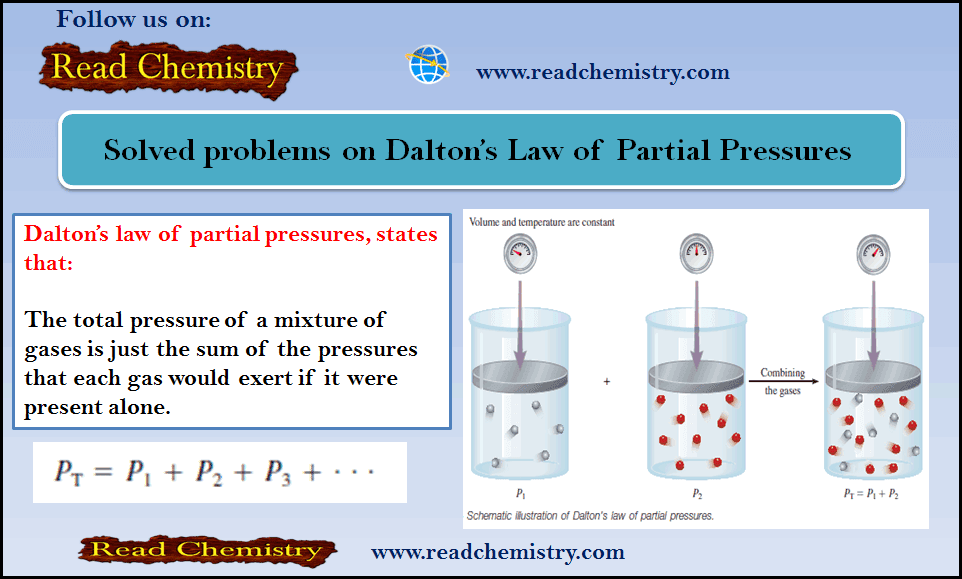
Solved problems: Dalton’s Law of Partial Pressures
– Before you solve these problems, you can read this subject for Dalton’s Law of Partial Pressures (Statement, Mathematical, Importance,…
Read More » -
General Chemistry
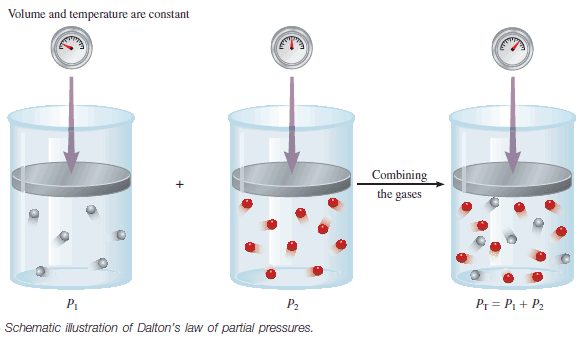
Dalton’s Law of Partial Pressures (Statement, Applications)
– In this subject, we will discuss Dalton’s Law of Partial Pressures (Statement, Mathematical, Importance, Application). Dalton’s Law of Partial Pressures…
Read More » -
Physical Chemistry
Hybridization and Shapes of Molecules
Hybridization and Shapes of Molecules – In the previous subject, we talked about the concept of Hybridization and the types…
Read More » -
General Chemistry
Density of gas: Definition, Equation, Solved Examples
– In this subject, we will discuss the Density of gas (Definition, Equation, Solved Examples) The Density of gas –…
Read More » -
General Chemistry
Molar Mass of gas: Definition, Equation, Solved Examples
– In this subject, we will discuss the Molar Mass of gas (Definition, Equation, Solved Examples) The Molar Mass of…
Read More » -
Organic Chemistry
Hybridization: Definition, Types, Rules, Examples
– In this subject, we will discuss the Hybridization: Definition, Types, Rules, and Examples – While the formation of simple…
Read More » -
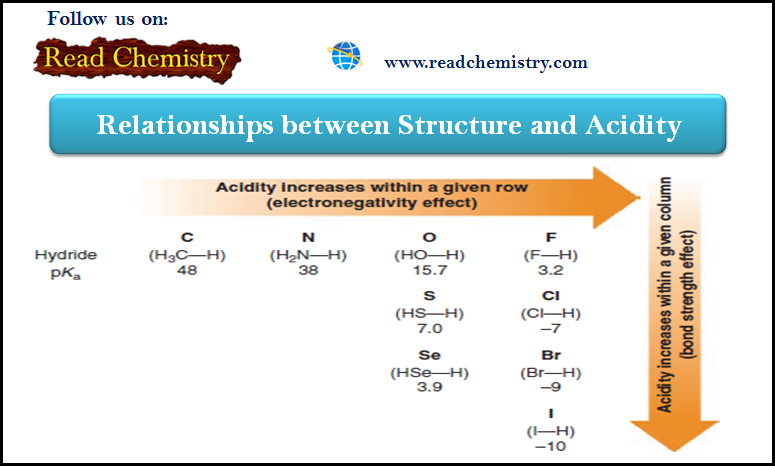
General Chemistry
Acidity: The relationship between Structure and Acidity
– In this subject, we will discuss the Relationships between Structure and Acidity. – The strength of a Brønsted–Lowry acid…
Read More » -
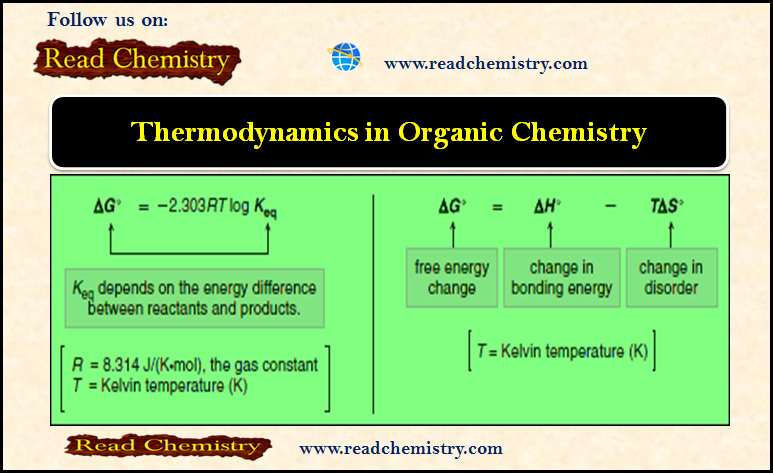
Organic Chemistry
Thermodynamics of Organic Compounds
– In this subject, we will discuss the Thermodynamics of Organic Compounds Thermodynamics of Organic Compounds – For a reaction…
Read More » -
General Chemistry
How to Predict the Outcome of acid-base reaction
– In this subject, we will discuss How to Predict the Outcome of acid-base reaction. How To Predict the Outcome…
Read More » -
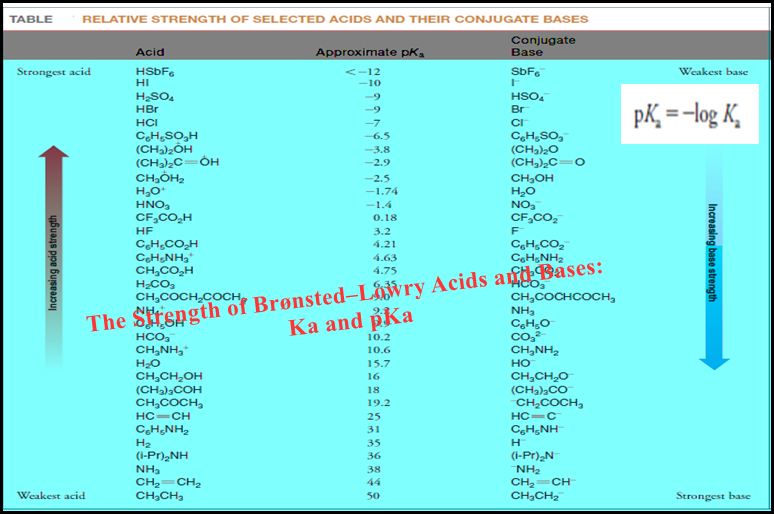
Analytical Chemistry
Bronsted-Lowry Acid Strength: Ka and pKa
– In this subject, we will discuss the Bronsted-Lowry Acid Strength: Ka and pKa Bronsted-Lowry Acid Strength: Ka and pKa…
Read More » -
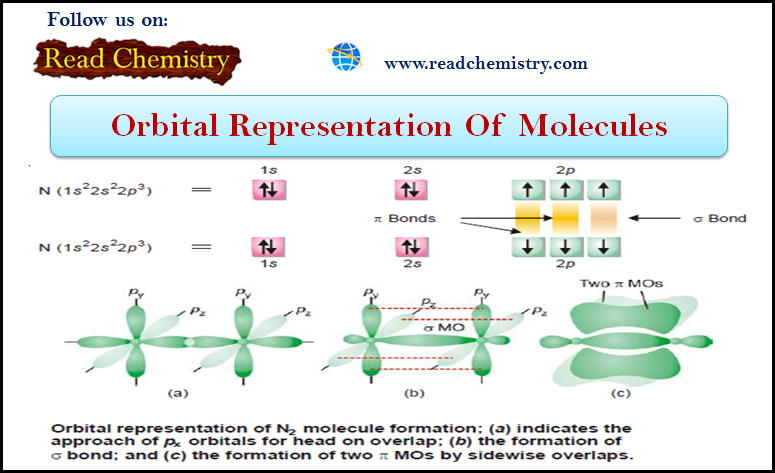
General Chemistry
Orbital Representation of Molecules
– In this subject, we will discuss the Orbital Representation of some Molecules (1) Orbital Representation of H2 molecule –…
Read More » -
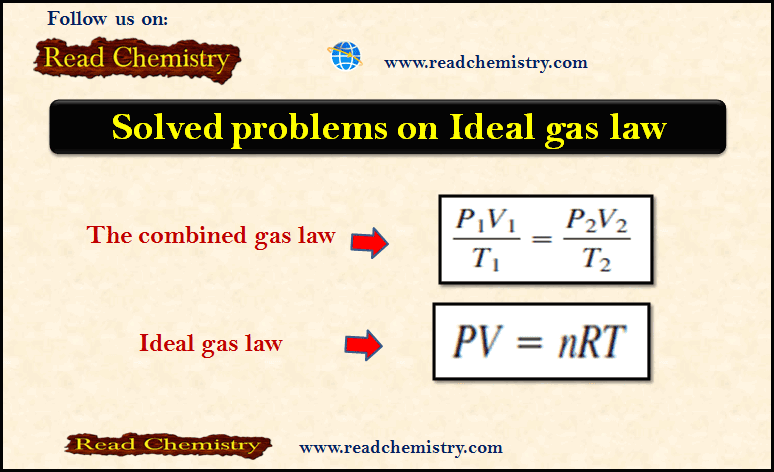
General Chemistry
The Ideal gas law: Solved Problems
– In this subject, we will discuss Solved Problems on The Ideal gas law Problem (1) on The ideal gas…
Read More » -
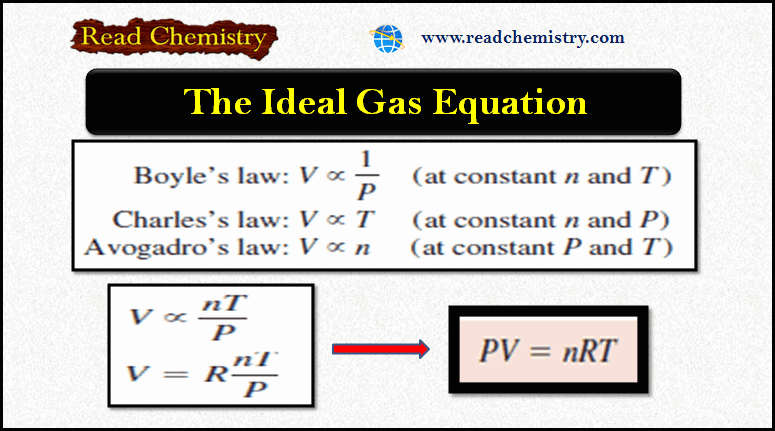
General Chemistry
Ideal Gas Equation: Definition, Formula, Notes
– In this subject, we will discuss the Ideal Gas Equation (Definition, Formula, Notes) The Ideal Gas Equation – Let…
Read More » -
Free book
Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition by Robert G. Mortimer The…
Read More »