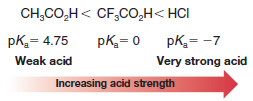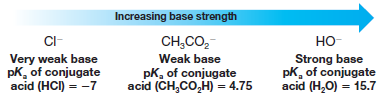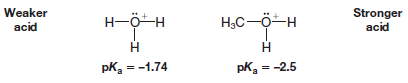Bronsted-Lowry Acid Strength: Ka and pKa
– In this subject, we will discuss the Bronsted-Lowry Acid Strength: Ka and pKa
Bronsted-Lowry Acid Strength: Ka and pKa
– Many organic reactions involve the transfer of a proton by an acid–base reaction.
– An important consideration, therefore, is the relative strengths of compounds that could potentially act as Brønsted–Lowry acids or bases in a reaction.
– In contrast to the strong acids, such as HCl and H2SO4, acetic acid is a much weaker acid.
– When acetic acid dissolves in water, the following reaction does not proceed to completion:
– Experiments show that in a 0.1 M solution of acetic acid at 25 oC only about 1% of the acetic acid molecules ionize by transferring their protons to water. Therefore, acetic acid is a weak acid.
– Acid strength is characterized in terms of acidity constant (Ka) or pKa values.
The Acidity Constant, Ka
– Because the reaction that occurs in an aqueous solution of acetic acid is an equilibrium, we can describe it with an expression for the equilibrium constant (Keq):
– For dilute aqueous solutions, the concentration of water is essentially constant (≈55.5 M), so we can rewrite the expression for the equilibrium constant in terms of a new constant (Ka) called the acidity constant:
– At 25 oC, the acidity constant for acetic acid is 1.76 × 10-5.
– We can write similar expressions for any weak acid dissolved in water.
– Using a generalized hypothetical acid (HA), the reaction in water is:
– and the expression for the acidity constant is:
– Because the concentrations of the products of the reaction are written in the numerator and the concentration of the undissociated acid in the denominator, a large value of Ka means the acid is a strong acid, and a small value of Ka means the acid is a weak acid.
– If the Ka is greater than 10, the acid will be, for all practical purposes, completely dissociated in water at concentrations less than 0.01 M.
Acidity and pKa
– Chemists usually express the acidity constant, Ka, as its negative logarithm, pKa:
– This is analogous to expressing the hydronium ion concentration as pH:

– For acetic acid the pKa is 4.75:
– Notice that there is an inverse relationship between the magnitude of the pKaand the strength of the acid.
– The larger the value of the pKa, the weaker is the acid.
– For example, acetic acid with pKa = 4.75 is a weaker acid than trifluoroacetic acid with pKa = 0 (Ka = 1).
– Hydrochloric acid with pKa= -7 (Ka = 107) is a far stronger acid than trifluoroacetic acid.
– It is understood that a positive pKa is larger than a negative pKa.
– Water, itself, is a very weak acid and undergoes self-ionization even in the absence of acids and bases:
– In pure water at 25 oC, the concentrations of hydronium and hydroxide ions are equal to 10-7 M.
– Since the concentration of water in pure water is 55.5 M, we can calculate the Ka for water.
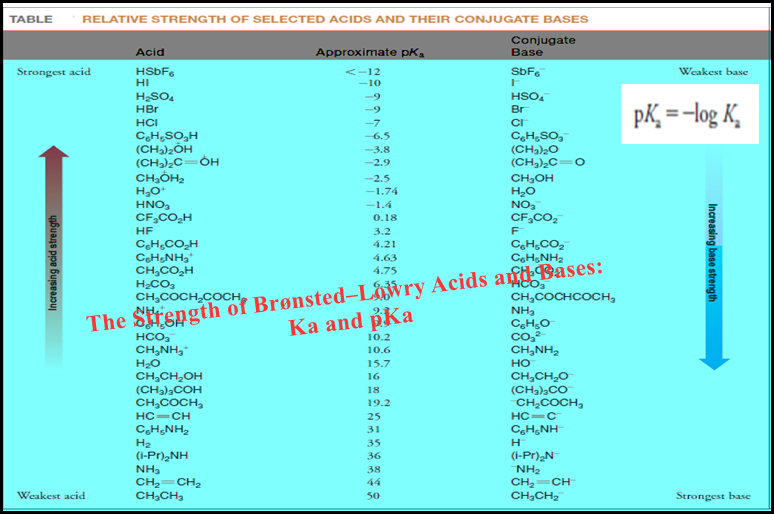
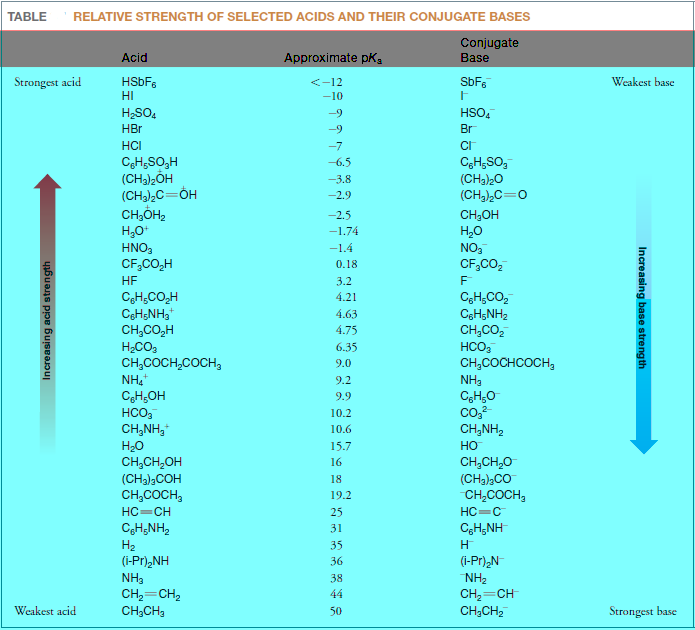
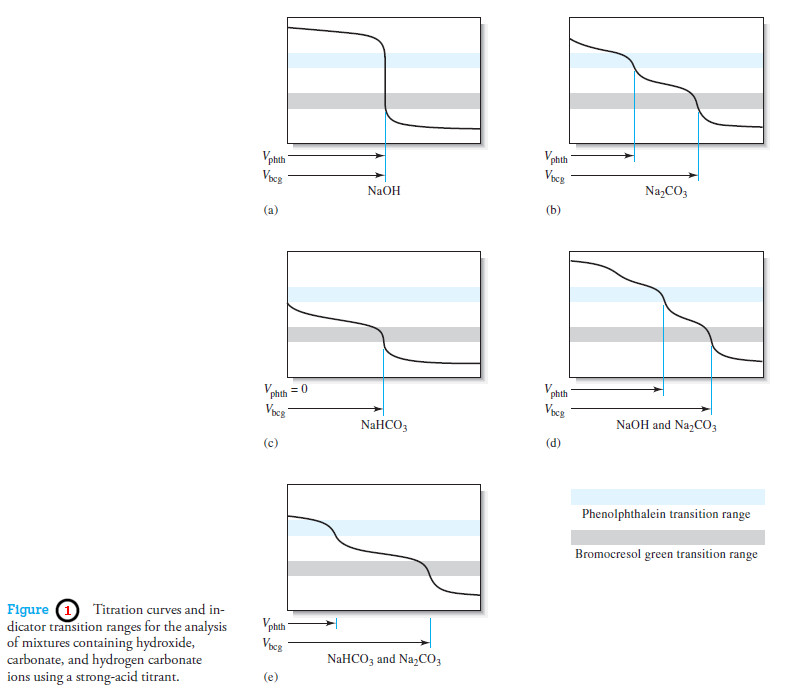
Lists pKa values
– The following Table shows lists pKa values for a selection of acids relative to water as the base.
– The values in the middle pKa range of the Table are the most accurate because they can be measured in aqueous solution.
– Special methods must be used to estimate the pKa values for the very strong acids at the top of the table and for the very weak acids at the bottom.
– The pKavalues for these very strong and weak acids are therefore approximate.
– Ethane is an extremely weak acid and HSbF6 is an acid that is so strong that it is called a superacid.
Predicting the Strength of Bases
– In our discussion so far we have dealt only with the strengths of acids.
– Arising as a natural corollary to this is a principle that allows us to estimate the base strength.
– Simply stated, the principle is this:
(1) The stronger the acid, the weaker will be its conjugate base. We can, therefore, relate the strength of a base to the pKa of its conjugate acid.
(2) The larger the pKa of the conjugate acid, the stronger is the base.
Consider the following as examples:
– We see that the hydroxide ion is the strongest in this series of three bases because its conjugate acid, water, is the weakest acid.
– We know that water is the weakest acid because it has the largest pKa.
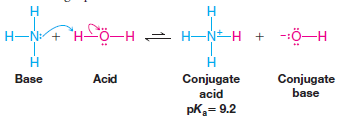
– Amines are like ammonia in that they are weak bases. Dissolving ammonia in water brings about the following equilibrium:
– Dissolving methylamine in water causes the establishment of a similar equilibrium.
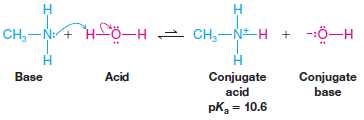
– Again we can relate the basicity of these substances to the strength of their conjugate acids.
– The conjugate acid of ammonia is the ammonium ion, NH4 +.
– The pKa of the ammonium ion is 9.2. The conjugate acid of methylamine is the CH3NH3+ ion. This ion, called the methylaminium ion, has pKa = 10.6.
– Since the conjugate acid of methylamine is a weaker acid than the conjugate acid of ammonia, we can conclude that methylamine is a stronger base than ammonia.
Solved Problems on Strength of Acid: Ka and pKa
Problem (1): Phenol (C6H5)OH has Ka = 1.26 × 10-10.
(a) What is the molar concentration of hydronium ion in a 1.0 M solution of phenol?
(b) What is the pH of the solution?
Strategy and answer:
– Use the equation for Ka for the equilibrium:
– At equilibrium, the concentration of hydronium ion will be the same as that of phenoxide ion, thus we can let them both equal (x) Therefore:
Problem (2): Show calculations proving that the pKa of the hydronium ion (H3O+) is -1.74
Strategy and answer:
– When H3O+acts as an acid in aqueous solution, the equilibrium is:
and Ka is equal to the molar concentration of water;
– The molar concentration of H2O in pure H2O is equal to the number of moles of H2O (MW = 18 g/mol) in 1000 g (one liter) of water.
– That is,
Problem (3): Using the pKa values in the Table shown above, decide which is the stronger base, CH3OH or H2O.
Strategy and Answer:
– From the Table shown above, we find the pKavalues of the conjugate acids of water and methanol.
– Because water is the conjugate base of the weaker acid, it is the stronger base.
Problem (4): Which would be the stronger base, HO– or NH3?
Strategy and answer:
– The conjugate acid of the hydroxide ion (HO–) is H2O, and water has pKa = 15.7 (Table).
– The conjugate acid of ammonia is the ammonium ion NH4+, which has pKa = 9.2 (meaning it is a stronger acid than water).
– Since the ammonium ion is the stronger acid, its conjugate base NH3 is the weaker base, and HO–, the conjugate base of water (the weaker acid), is the stronger base.
Reference: Organic chemistry / T.W. Graham Solomons , Craig B.Fryhle , Scott A.snyder , / ( eleventh edition) / 2014.