– In this subject, we will discuss the Gaseous Substances: Substances That Exist as Gases Gaseous Substances – Under certain conditions of pressure and temperature, most substances can exist in any one of three states of matter: solid, liquid, or gas. – Water, for example, can be solid ice, liquid …
Read More »Dilution of Solutions
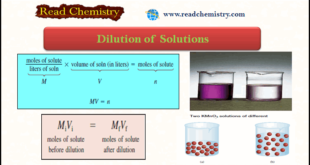
– In this subject, we will discuss the Dilution of Solutions and Dilution Law. Dilution of Solutions – Concentrated solutions are often stored in the laboratory stockroom for use as needed. – Frequently we dilute these “stock” solutions before working with them. – Dilution of solution is the procedure for …
Read More »50 MCQ on Chemical bonding
MCQ on Chemical bonding – In this subject, you will find 50 questions and answers MCQ on Chemical bonding 1. The valency of an element is ___________ (a) the combining capacity of one atom of it (b) the number of bonds formed by its one atom( c) the number …
Read More »Molarity: definition, formula, solved Problems
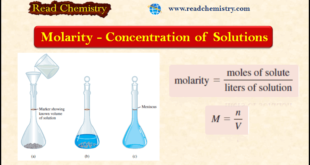
– In this subject, we will discuss the Molarity: definition, formula, solved Problems – To study solution stoichiometry, we must know how much of the reactants are present in a solution and also how to control the amounts of reactants used to bring about a reaction in aqueous solution. The …
Read More »VSEPR Theory: Postulates, Predicting Shapes of Molecules
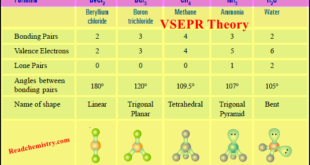
– In this subject, we will discuss the VSEPR Theory: definition, Postulates, limitations, and Predicting Shapes of Molecules. VSEPR Theory – The Lewis structure of a molecule tells us the number of pairs of electrons in the valence shell of the central atom. – These electron pairs are subject to …
Read More »Redox Reactions: Types, Examples, Applications, Balancing
– In this subject, we will discuss the Redox Reactions: Types, Examples, Applications, Balancing. Types of Redox Reactions – Among the most common oxidation-reduction reactions are: Combination Reactions Decomposition Reactions Combustion Reactions Displacement Reactions Disproportionation Reaction (1) Combination Reactions – A combination reaction is a reaction in which two or …
Read More »Bond Dissociation Energy: Definition, Equation, Problems
– In this subject, we will discuss the Bond Dissociation Energy: Definition, Equation, Problems Bond Dissociation Energy – Bond breaking can be quantified using the bond dissociation energy. – The bond dissociation energy is the energy needed to homolytically cleave a covalent bond. – The energy absorbed or released in …
Read More »Acid-Base Reactions: Definition, Examples, and Uses
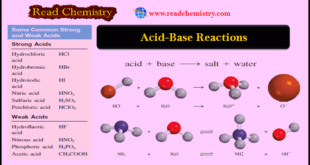
– In this subject, we will discuss the Acid-Base Reactions: Definition, Examples, and Uses – Acids and bases are as familiar as aspirin and milk of magnesia although many people do not know their chemical names—acetylsalicylic acid (aspirin) and magnesium hydroxide (milk of magnesia). – In addition to being the …
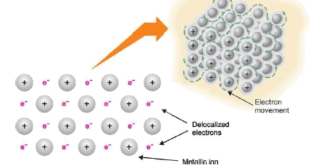
Read More »Metallic Bonding: Definition, Properties, Examples, Explanation
– In this subject, we will discuss the Metallic Bonding: Definition, Properties, Examples, Explanation Metallic Bonding – The valence bonds that hold the atoms in a metal crystal together are not ionic, nor are they simply covalent in nature. – Ionic bonding is obviously impossible here since all the atoms …
Read More »Precipitation Reactions: Definition and Examples
– In this subject, we will discuss the Precipitation Reactions: Definition and Examples What are Precipitation Reactions? – One common type of reaction that occurs in an aqueous solution is the precipitation reaction, which results in the formation of an insoluble product, or precipitate. – A precipitate is an insoluble …
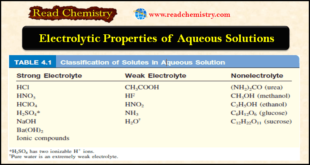
Read More »Aqueous Solutions: Definition, Examples, Electrolytic Properties
– In this subject, we will discuss the Aqueous Solutions: Definition, Examples, Electrolytic Properties Difference between Solution, solute, solvent – A solution is a homogeneous mixture of two or more substances. – The solute is the substance present in a smaller amount, and the solvent is the substance present in …
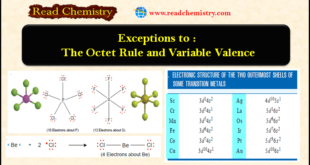
Read More »Exceptions to the Octet Rule and Variable Valence
Exceptions to the octet rule – For a time it was believed that all compounds obeyed the Octet rule or the Rule of Eight. – However, it gradually became apparent that quite a few molecules had non-octet structures. – Atoms in these molecules could have number of electrons in the …
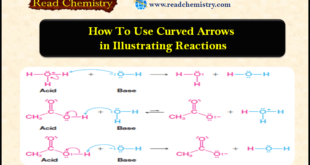
Read More »How to use Curved Arrows in illustrating Reactions
– In this subject, we will discuss How to use Curved Arrows in illustrating Reactions. How to use Curved Arrows in illustrating reactions – Up to this point, we have not indicated how bonding changes occur in the reactions we have presented, but this can easily be done using curved-arrow …
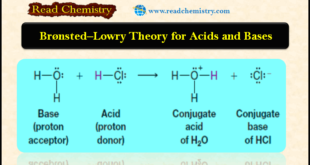
Read More »Bronsted-Lowry theory for acids and bases
– In this subject, we will discuss Bronsted-Lowry theory for acids and bases Acid-base reactions – We begin our study of chemical reactions and mechanisms by examining some of the basic principles of acid-base chemistry. – There are several reasons for doing this: (1) Many of the reactions that occur …
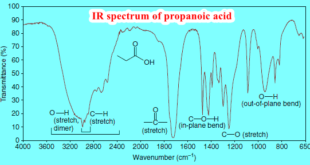
Read More »How to interpret IR spectrum without Knowledge of the structure
– In this subject, we will discuss How to interpret an IR spectrum without any Knowledge of the structure How to interpret IR Spectrum without any Knowledge of the structure – IR spectroscopy is an incredibly powerful tool for functional group identification, as we have seen in the preceding subjects. …
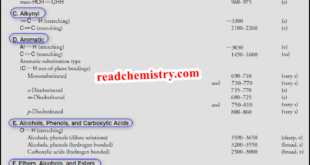
Read More »Interpreting IR Spectra
Interpreting IR Spectra – IR spectra contain a wealth of information about the structures of compounds. – We show some of the information that can be gathered from the spectra of octane and methylbenzene (commonly called toluene) in Figs.1 and 2. – In this subject, we shall learn how to …
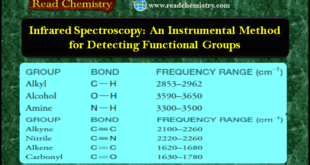
Read More »Infrared Spectroscopy: Instrumental Method for Detecting Functional Groups
– In this subject, we will discuss Infrared Spectroscopy: Instrumental Method for Detecting Functional Groups. Infrared Spectroscopy – Infrared spectroscopy (IR spectroscopy ): is a simple, rapid, and nondestructive instrumental technique that can give evidence for the presence of various functional groups. – If you had a sample of unknown …
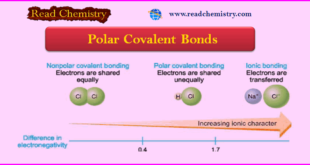
Read More »Polar Covalent Bond: Definition, Properties, Examples
– In this subject, we will discuss the Polar Covalent Bond: Definition, Properties, Examples Polar Covalent Bonds – In the H2 or Cl2 molecule, the two electrons constituting the covalent bond are equally shared by the two identical nuclei. – Due to the even distribution of (+) and (–) charge, …
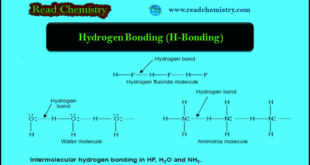
Read More »Hydrogen Bonding: Definition, types, Examples, Characteristics
– In this subject, we will discuss the hydrogen Bonding (Definition, types, Examples, Characteristics) Hydrogen Bonding (H-Bonding) – When hydrogen (H) is covalently bonded to a highly electronegative atom X (O, N, F), the shared electron pair is pulled so close to X that a strong dipole results – Since the …
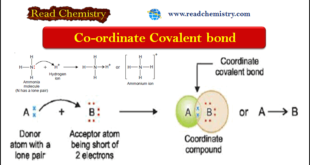
Read More »Coordinate Bond: Definition, Formation, Examples
– In this subject, we will discuss the Coordinate Bond: (Definition, Formation, Examples) Coordinate bond – In a normal covalent bond, each of the two bonded atoms contributes one electron to make the shared pair. – In some cases, a covalent bond is formed when both electrons are supplied entirely …
Read More » Read Chemistry
Read Chemistry