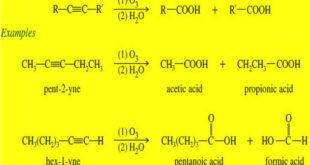
Before we discuss Oxidation of Alkynes we will talk about triple bond of Alkynes What are Alkynes? – Alkynes are hydrocarbons that contain carbon–carbon triple bonds. – Alkynes are also called acetylenes because they are derivatives of acetylene, the simplest alkyne – The chemistry of the carbon–carbon triple bond is …
Read More »Addition Reactions of Alkynes
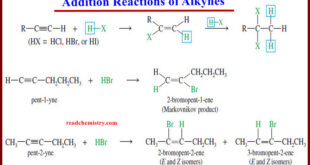
Addition Reactions of Alkynes – Many of the reactions of alkynes are similar to the corresponding reactions of alkenes because both involve pi bonds between two carbon atoms. – Like the pi bond of an alkene, the pi bonds of an alkyne are electron-rich, and they readily undergo addition reactions. …
Read More »Synthesis of alkynes
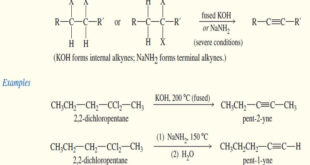
Synthesis of alkynes – Two different approaches are commonly used for the synthesis of alkynes. – In the first, an appropriate electrophile undergoes nucleophilic attack by an acetylide ion. – The electrophile may be an unhindered primary alkyl halide (undergoes SN2), or it may be a carbonyl compound (undergoes addition …
Read More »Acidity of Alkynes : Formation of Acetylide Ions
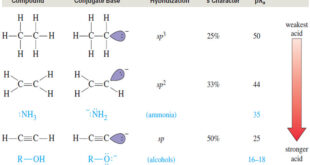
Acidity of Alkynes: Formation of Acetylide Ions – Acidity of Alkynes is the inportant factor of activity of Alkynes. – Terminal alkynes are much more acidic than other hydrocarbons. – Removal of an acetylenic proton forms an acetylide ion, which plays a central role in alkyne chemistry. – The acidity …
Read More »The electronic structure of Alkynes
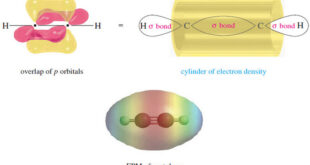
– we studied theThe electronic structureof Alkynes (a triple bond) in This subject: The Structure of Ethyne (Acetylene): sp Hybridization – Let’s review The electronic structureof Alkynes, using acetylene as the example. – The Lewis structure of acetylene shows three pairs of electrons in the region between the carbon nuclei: …
Read More »Importance of Alkynes : Acetylene
Commercial Importance of Alkynes : Acetylene and Methylacetylene Uses of Acetylene and Methylacetylene – Acetylene is by far the most important commercial alkyne. – and Acetylene is an important industrial feedstock, but its largest use is as the fuel for the oxyacetylene welding torch. – It is a colorless, foul-smelling …
Read More »Nomenclature of Alkynes
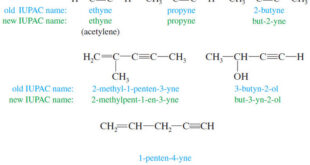
What are Alkynes? – Alkynes are hydrocarbons that contain carbon–carbon triple bonds. – Alkynes are also called acetylenes because they are derivatives of acetylene, the simplest alkyne – The chemistry of the carbon–carbon triple bond is similar to that of the double bond. – Alkynes undergo most of the same …
Read More »Reactions of Alkenes
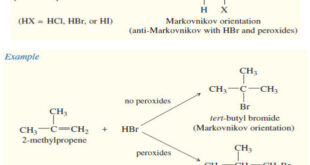
– In this subject we will discuss all famous reactions of alkenes. – The reactions of alkenes include five basic methods as follow: (1) Electrophilic Additions (a) Addition of hydrogen halides – A Russian chemist, Vladimir Markovnikov, first showed the orientation of addition of HBr to alkenes in 1869. – …
Read More »Olefin Metathesis
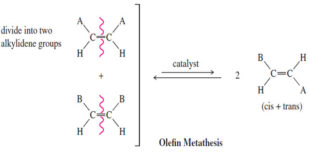
Olefin Metathesis – The double bond is the strongest bond in an alkene, yet it is also the most reactive bond. – Imagine how useful it would be if we could break molecules at their double bonds and reassemble them as we please. That is the goal of olefin metathesis. …
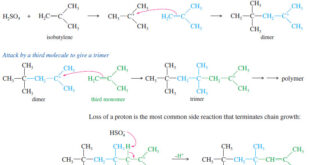
Read More »Polymerization of Alkenes
Polymerization of Alkenes – A polymer is a large molecule composed of many smaller repeating units (the monomers) bonded together. – Alkenes serve as monomers for some of the most common polymers, such as polyethylene, polypropylene, polystyrene, poly(vinyl chloride), and many others. – Alkenes polymerize to give addition polymers resulting …
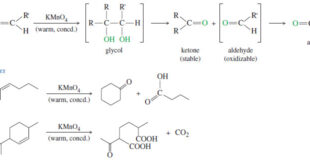
Read More »Oxidative Cleavage of Alkenes
Oxidative Cleavage of Alkenes Cleavage by Permanganate – In a potassium permanganate dihydroxylation, if the solution is warm or acidic or too concentrated, oxidative cleavage of the glycol may occur. – In effect, the double bond is cleaved to two carbonyl groups. – The products are initially ketones and aldehydes, …
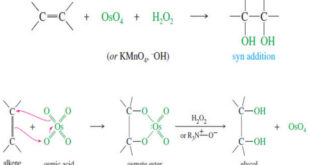
Read More »Syn Dihydroxylation of Alkenes
Syn Dihydroxylation of Alkenes – Converting an alkene to a glycol requires adding a hydroxyl group to each end of the double bond. This addition is called Dihydroxylation of Alkenes or dihydroxylation (or hydroxylation) of the double bond. – We have seen that epoxidation of an alkene, followed by acidic …
Read More »Chemical Bonding – Orbital Concept – MCQ test
Online MCQ test on Chemical Bonding – Orbital Concept – In this topic we offer you, online MCQ test in the fundmental of Chemical Bonding – Orbital Concept. – It is a simple test to measure the strength of your knowledge in the basics of understanding the topics of Chemical …
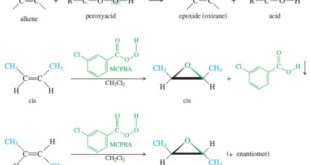
Read More »Epoxidation of Alkenes
Epoxidation of Alkenes – Some of the most important reactions of alkenes involve oxidation. – When we speak of oxidation, we usually mean reactions that form carbon–oxygen bonds. (Halogens are oxidizing agents, and the addition of a halogen molecule across a double bond is formally an oxidation as well.) – …
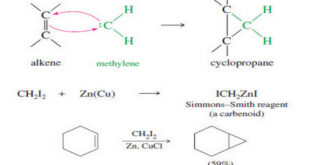
Read More »Addition of Carbenes to Alkenes
Addition of Carbenes to Alkenes – Methylene (:CH2) is the simplest of the carbenes: uncharged, reactive intermediates that have a carbon atom with two bonds and two nonbonding electrons. – Like borane (BH3), methylene is a potent electrophile because it has an unfilled octet. – It adds to the electronrich …
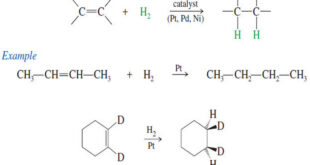
Read More »Catalytic Hydrogenation of Alkenes
Catalytic Hydrogenation of Alkenes – Although we have mentioned catalytic hydrogenation before, we now consider the mechanism and stereochemistry in more detail. – Hydrogenation of an alkene is formally a reduction, with H2 adding across the double bond to give an alkane. – The process usually requires a catalyst containing …
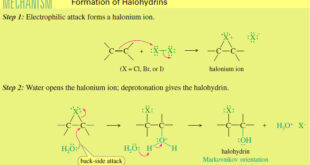
Read More »Formation of Halohydrin
Formation of Halohydrin – A halohydrin is an alcohol with a halogen on the adjacent carbon atom. – In the presence of water, halogens add to alkenes to form halohydrins. – The electrophilic halogen adds to the alkene to give a halonium ion, which is also electrophilic. – Water acts …
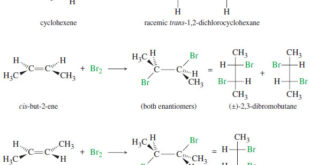
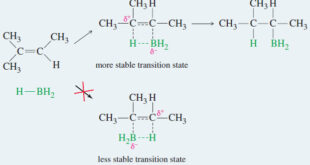
Read More »Addition of Halogens to Alkenes
Addition of Halogens to Alkenes – Addition of Halogens to Alkenes gives vicinal dihalides. (A) Mechanism of Halogen Addition – A halogen molecule (Br2, Cl2, or I2) is electrophilic; a nucleophile can react with a halogen, displacing a halide ion: – In this example, the nucleophile attacks the electrophilic nucleus …
Read More »Hydroboration of Alkenes
Hydroboration of Alkenes – We have seen two methods for hydrating an alkene with Markovnikov orientation. – What if we need to convert an alkene to the anti-Markovnikov alcohol? – For example, the following transformation cannot be accomplished using the hydration procedures covered thus far. – Such an anti-Markovnikov hydration …
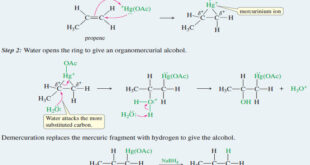
Read More »Oxymercuration–demercuration of alkenes
– Oxymercuration–demercuration of alkenes is another method for converting alkenes to alcohols with Markovnikov orientation. Hydration of alkenes by Oxymercuration–Demercuration – Many alkenes do not easily undergo hydration in aqueous acid. – Some alkenes are nearly insoluble in aqueous acid, and others undergo side reactions such as rearrangement, polymerization, or …
Read More » Read Chemistry
Read Chemistry