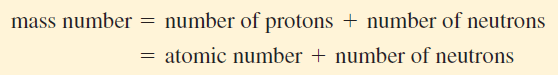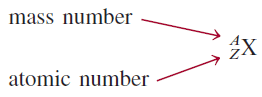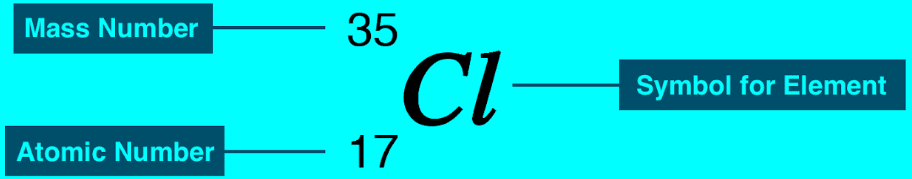Atomic Number, Mass Number, and Isotopes
– In this subject, we will discuss the Atomic Number, Mass Number, and Isotopes: Definition, Formula, and Calculation
Atomic number
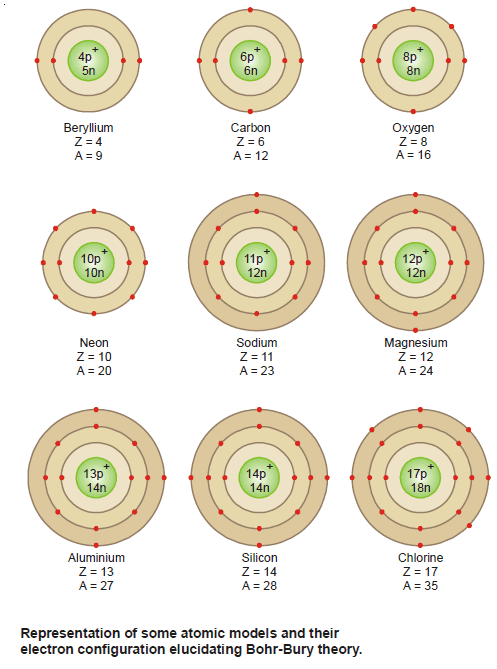
– All atoms can be identified by the number of protons and neutrons they contain.
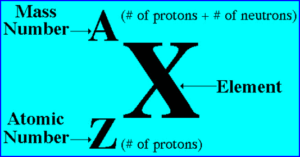
– The number of protons in the nucleus of each atom of an element is called the atomic number (Z).
– In a neutral atom, the number of protons is equal to the number of electrons, so the atomic number also indicates the number of electrons present in the atom.
– The chemical identity of an atom can be determined solely by its atomic number.
– For example, the atomic number of nitrogen is 7; this means that each neutral nitrogen atom has 7 protons and 7 electrons.
– Or viewed another way, every atom in the universe that contains 7 protons is correctly named “nitrogen.”
Mass number
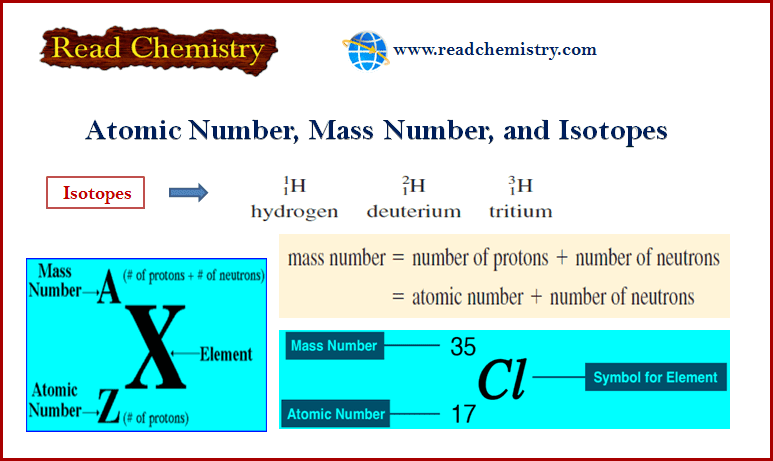
– The mass number (A) is the total number of neutrons and protons present in the nucleus of an atom of an element.
– Except for the most common form of hydrogen, which has one proton and no neutrons, all atomic nuclei contain both protons and neutrons.
– In general, the mass number is given by:
– The number of neutrons in an atom is equal to the difference between the mass number and the atomic number, or (A – Z).
– For example, if the mass number of a particular boron atom is 12 and the atomic number is 5 (indicating 5 protons in the nucleus), then the number of neutrons is 12 – 5 = 7.
– Note that all three quantities (atomic number, number of neutrons, and mass number) must be positive integers or whole numbers.
– The accepted way to denote the atomic number and mass number of an atom of element X is as follows:
Isotopes
– In most cases, atoms of a given element do not all have the same mass.
– Atoms that have the same atomic number but different mass numbers are called isotopes.
– For example, there are three isotopes of hydrogen. One, simply known as hydrogen, has one proton and no neutrons.
– The deuterium isotope has one proton and one neutron, and tritium has one proton and two neutrons.
– Thus, for the isotopes of hydrogen, we write:
– As another example, consider two common isotopes of uranium with mass numbers of 235 and 238, respectively:
– The first isotope is used in nuclear reactors and atomic bombs, whereas the second isotope lacks the properties necessary for these applications.
– Except for hydrogen, isotopes of elements are identified by their mass numbers.
– Thus, these two isotopes are called uranium-235 (pronounced “uranium two thirty-five”) and uranium-238 (pronounced “uranium two thirty-eight”).
– The chemical properties of an element are determined primarily by the protons and electrons in its atoms; neutrons do not take part in chemical changes under normal conditions.
– Therefore, isotopes of the same element have similar chemistries, forming the same types of compounds and displaying similar reactivities.
Solved problem on Atomic number
Give the number of protons, neutrons, and electrons in each of the following species:
(a) 179Au95 , (b) 179Au97 , (c) 18F , (d) carbon-13.
Solution:
(a) The atomic number of Au (gold) is 79, so there are 79 protons.
– The mass number is 195, so the number of neutrons is 195 – 79 = 116.
– The number of electrons is the same as the number of protons; that is, 79.
(b) Here the number of protons is the same as in (a), or 79.
– The mass number is 197, so the number of neutrons is 197 – 79 = 118.
– The number of electrons is also the same as in (a), 79.
– The species in (a) and (b) are chemically similar isotopes of gold.
(c) The atomic number of F (fluorine) is 9, so there are 9 protons.
– The mass number is 18, so the number of neutrons is 18 – 9 = 9.
– The number of electrons is the same as the number of protons; that is, 9.
(d) Carbon-13 can also be represented as 13C.
– The atomic number of carbon is 6, so there are 13 – 6 = 7 neutrons.
– The number of electrons is 6.
Reference: General Chemistry – The Essential Concepts / Raymond Chang, Jason Overby. ( sixth edition) .