Lewis Structures: Definition, Structural Formula, Examples
– In this subject, we will discuss the Lewis Structures: Definition, Overview, Structural Formula, Examples
Definition of Lewis structures
– A Lewis structure is a structural representation of a molecule where dots are used to show electron positions around the atoms and lines or dot pairs represent covalent bonds between atoms.
– The purpose of drawing a Lewis dot structure is to identify the lone electron pairs in molecules to help determine chemical bond formation.
– Lewis structures can be made for molecules that contain covalent bonds and for coordination compounds.
– The reason is that electrons are shared in a covalent bond. In an ionic bond, it’s more like one atom donates an electron to the other atom.
– Lewis structures are named for Gilbert N. Lewis, who introduced the idea in the article “The Atom and the Molecule” in 1916.
How to write Lewis structures
– There are several simple rules that allow us to draw proper Lewis structures:
Rule (1) for writing Lewis structures
(1) Lewis structures show the connections between atoms in a molecule or ion using only the valence electrons of the atoms involved.
Valence electrons are those of an atom’s outermost shell.
Rule (2) for writing Lewis structures
(2) For main group elements, the number of valence electrons a neutral atom brings to a Lewis structure is the same as its group number in the periodic table.
– Carbon, for example, is in group IVA and has four valence electrons; the halogens (e.g., fluorine) are in group VIIA and each has seven valence electrons; hydrogen is in group IA and has one valence electron.
Rule (3)
(3) If the structure we are drawing is a negative ion (an anion), we add one electron for each negative charge to the original count of valence electrons.
– If the structure is a positive ion (a cation), we subtract one electron for each positive charge.
Rule (4)
(4) In drawing Lewis structures we try to give each atom the electron configuration of a noble gas.
– To do so, we draw structures where atoms share electrons to form covalent bonds or transfer electrons to form ions.
(a) Hydrogen forms one covalent bond by sharing its electron with an electron of another atom so that it can have two valence electrons, the same number as in the noble gas helium.
(b) Carbon forms four covalent bonds by sharing its four valence electrons with four valence electrons from other atoms, so that it can have eight electrons (the same as the electron configuration of neon, satisfying the octet rule).
(c) To achieve an octet of valence electrons, elements such as nitrogen, oxygen, and halogens typically share only some of their valence electrons through covalent bonding, leaving others as unshared electron pairs.
Solved Problems
– The following problems illustrate the rules above.
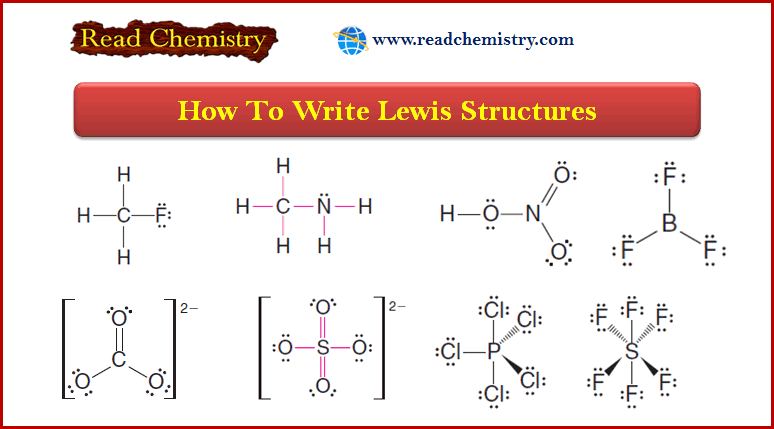
Solved problem (1): Write the Lewis structure of CH3F.
Strategy and Answer:
1. We find the total number of valence electrons of all the atoms:
2. We use pairs of electrons to form bonds between all atoms that are bonded to each other.
– We represent these bonding pairs with lines.
– In our example, this requires four pairs of electrons (8 of the 14 valence electrons).
3. We then add the remaining electrons in pairs to give each hydrogen 2 electrons (a duet) and every other atom 8 electrons (an octet).
– In our example, we assign the remaining 6 valence electrons to the fluorine atom in three nonbonding pairs.
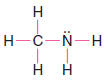
Solved problem (2): Write a Lewis structure for methylamine (CH3NH2).
Strategy and Answer:
1. We find the total number of valence electrons for all the atoms.
2. We use one electron pair to join the carbon and nitrogen.
3. We use three pairs to form single bonds between the carbon and three hydrogen atoms.
4. We use two pairs to form single bonds between the nitrogen atom and two hydrogen atoms.
5. This leaves one electron pair, which we use as a lone pair on the nitrogen atom.
Rule (5)
(5) If necessary, we use multiple bonds to satisfy the octet rule (i.e., give atoms the noble gas configuration).
– The carbonate ion (CO32-) illustrates this:
– The organic molecules ethene (C2H4) and ethyne (C2H2), as mentioned earlier, have a double and triple bond, respectively:
Solved problem (3): Write the Lewis structure of CH2O (formaldehyde).
Strategy and Answer:
1. Find the total number of valence electrons of all the atoms:
2. (a) Use pairs of electrons to form single bonds
(b) Determine which atoms already have a full valence shell and which ones do not, and how many valence electrons we have used so far.
– In this case, we have used 6 valence electrons, and the valence shell is full for the hydrogen atoms but not for the carbon and oxygen.
(c) We use the remaining electrons as bonds or unshared electron pairs, to fill the valence shell of any atoms whose valence shell is not yet full, taking care not to exceed the octet rule.
– In this case 6 of the initial 12 valence electrons are left to use.
– We use 2 electrons to fill the valence shell of the carbon by another bond to the oxygen, and the remaining 4 electrons as two unshared electron pairs with the oxygen, filling its valence shell.
Rule (6)
(6) Before we can write some Lewis structures, we must know how the atoms are connected to each other.
– Consider nitric acid, for example.
– Even though the formula for nitric acid is often written HNO3, the hydrogen is actually connected to oxygen, not to nitrogen.
– The structure is HONO2 and not HNO3.
– Thus the correct Lewis structure is:
Solved problem (4): Assume that the atoms are connected in the same way they are written in the formula, and write a Lewis structure for the toxic gas hydrogen cyanide (HCN).
Strategy and Answer:
1. We find the total number of valence electrons on all of the atoms:
2. We use one pair of electrons to form a single bond between the hydrogen atom and the carbon atom (see below), and we use three pairs to form a triple bond between the carbon atom and the nitrogen atom. This leaves two electrons.
– We use these as an unshared pair on the nitrogen atom.
– Now each atom has the electronic structure of a noble gas.
– The hydrogen atom has two electrons (like helium) and the carbon and nitrogen atoms each have eight electrons (like neon).
Exceptions to the Octet Rule
(1) Atoms share electrons, not just to obtain the configuration of inert gas, but because sharing electrons produces increased electron density between the positive nuclei.
– The resulting attractive forces of nuclei for electrons is the “glue” that holds the atoms together
– Elements of the second period of the periodic table can have a maximum of four bonds (i.e., have eight electrons around them) because these elements have only one 2s and three 2p orbitals available for bonding.
(2) Each orbital can contain two electrons, and a total of eight electrons fills these orbitals The octet rule, therefore, only applies to these elements, and even here, as we shall see in compounds of beryllium and boron, fewer than eight electrons are possible.
– Elements of the third period and beyond have d orbitals that can be used for bonding.
(3) These elements can accommodate more than eight electrons in their valence shells and therefore can form more than four covalent bonds.
– Examples are compounds such as PCl5 and SF6.
– Bonds written as (dashed wedges) project behind the plane of the paper.
– Bonds written as (solid wedges) project in front of the paper.
Solved Problem
Solved problem (5): Write a Lewis structure for the sulfate ion (SO42-) (Note: The sulfur atom is bonded to all four oxygen atoms.)
Strategy and Answer:
1. We find the total number of valence electrons including the extra 2 electrons needed to give the ion the double negative charge:
2. We use four pairs of electrons to form bonds between the sulfur atom and the four oxygen atoms
3. We add the remaining 24 electrons as unshared pairs on oxygen atoms and as double bonds between the sulfur atom and two oxygen atoms.
– This gives each oxygen 8 electrons and the sulfur atom 12:
(4) Some highly reactive molecules or ions have atoms with fewer than eight electrons in their outer shell.
– An example is boron trifluoride (BF3).
– In a BF3 molecule, the central boron atom has only six electrons around it:
Reference: Organic chemistry / T.W. Graham Solomons, Craig B.Fryhle, Scott A.snyder, / ( eleventh edition) / 2014

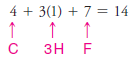





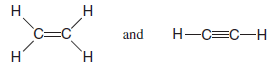
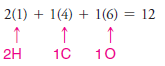


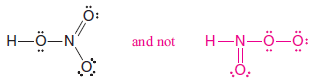
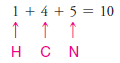





Organic chemistry
Inorganic chemistry
environmental chemistry