Charles’s Law: Relationship Between Temperature And Volume
– In this subject, we will discuss Charles’s Law: Relationship Between Temperature And Volume ( V-T relationship).
Relationship Between Temperature And Volume
– Boyle’s law depends on the temperature of the system remaining constant.
– But suppose the temperature changes: How does a temperature change affect the volume and pressure of a gas?
– Let us first look at the effect of temperature on the volume of a gas.
– The earliest investigators of this relationship were French scientists, Jacques Charles and Joseph Gay-Lussac.
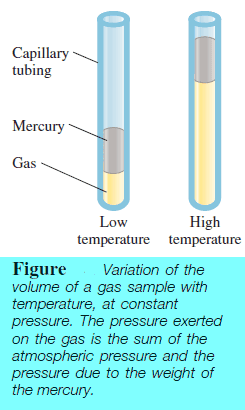
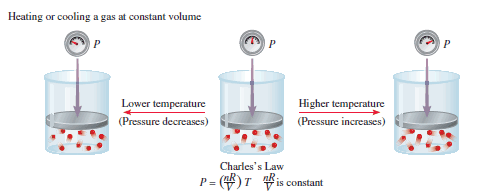
– Studies by Jacques Charles and Joseph Gay-Lussac showed that, at constant pressure, the volume of a gas sample expands when heated and contracts when cooled.
Absolute Zero, Kelvin temperature scale
– The quantitative relations involved in changes in gas temperature and volume turn out to be remarkably consistent.
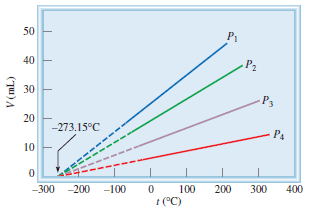
– For example, we observe an interesting phenomenon when we study the temperature-volume relationship at various pressures.
– At any given pressure, the plot of volume versus temperature yields a straight line.
– By extending the line to zero volume, we find the intercept on the temperature axis to be -273.15oC.
– At any other pressure, we obtain a different straight line for the volume-temperature plot, but we get the same zero-volume temperature intercept at -273.15oC.
– The previous figure shows the Variation of the volume of a gas sample with temperature, at constant pressure.
– Each line represents the variation at a certain pressure. The pressures increase from P1 to P4.
– All gases ultimately condense (become liquids) if they are cooled to sufficiently low temperatures; the solid portions of the lines represent the temperature region above the condensation point.
– When these lines are extrapolated, or extended (the dashed portions), they all intersect at the point representing zero volume and a temperature of -273.15oC.
– In practice, we can measure the volume of a gas over only a limited temperature range, because all gases condense at low temperatures to form liquids.
– In 1848 Lord Kelvin realized the significance of this phenomenon.
– He identified -273.15oC as absolute zero, theoretically the lowest attainable temperature.
– Then he set up an absolute temperature scale, now called the Kelvin temperature scale, with absolute zero as the starting point.
– On the Kelvin scale, one kelvin (K) is equal in magnitude to one degree Celsius.
Difference between the absolute temperature scale and the Celsius scale
– The only difference between the absolute temperature scale and the Celsius scale is that the zero position is shifted.
– Important points on the two scales match up as follows:
– The conversion between oC and K is given by the equation:
K = (°C + 273.15)

– By convention, we use T to denote absolute (kelvin) temperature and t to indicate temperature on the Celsius scale.
Charles’s law
– Charles’s law states that:
The volume of a fixed amount of gas maintained at constant pressure is directly proportional to the absolute temperature of the gas.
– The dependence of the volume of a gas on temperature is given by (Charles’s law):
where (k) is the proportionality constant.
– The previous Equation is known as Charles’s and Gay-Lussac’s law, or simply Charles’s law,
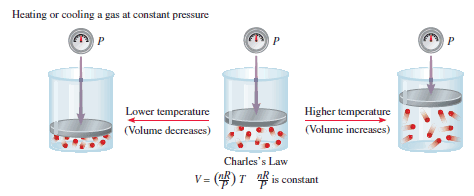
– Charles’s law is also illustrated in the following Figure:
– We see that the proportionality constant (k) in the previous Equation is equal to nR/P
– Just as we did for pressure-volume relationships at constant temperature, we can compare two sets of volume-temperature conditions for a given sample of gas at constant pressure.
– So we can write:
– where V1and V2 are the volumes of the gas at temperatures T1 and T2 (both in kelvins), respectively.
Another form of Charles’s law ( P-T relationship)
– Another form of Charles’s law shows that at a constant amount of gas and volume, the pressure of a gas is proportional to temperature.
– From the following Figure, we see that k = nR/V. So we have:
– where P1 and P2 are the pressures of the gas at temperatures T1 and T2 , respectively
Solved problems on Charles’s law
Example (1): Calculate the equivalent temperature on the other scale (Kelvin or Celsius) for each of the following:
Solution
Example (2): Show the data in the following Table:
prove:
(a) that the Celsius temperature is not directly proportional to volume and
(b) that the Kelvin temperature is directly proportional to volume.
Solution
(a) One obvious example: As the temperature is doubled the volume does not change. The volume is not directly proportional to Celsius temperature.
(b) As the absolute temperature 273 K is increased to 373 K or 473 K, the volume increases to 373/273 = 1.37 or 473/273 = 1.73 times the original volume. The ratio of V to T is constant (see Table). The volume is directly proportional to absolute temperature.
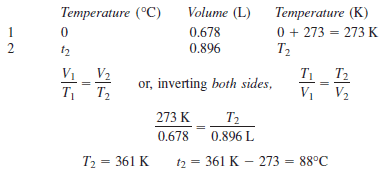
Example (3): Calculate the Celsius temperature to which a 678-mL sample of gas at 0 Co must be heated at constant pressure for the volume to change to 0.896 L.
Solution
– The data are tabulated, with 678 mL converted to 0.678 L:
Reference:
- Chemistry / Raymond Chang, Williams College /(10th edition).
- Fundamentals of Chemistry / David E.Goldberg/(5th edition).