Nuclear Chemistry Quiz: Questions and Answers
Nuclear Chemistry Quiz
– In this subject, you will find 40 questions and answers MCQ on Nuclear Chemistry
1. Which is the correct symbol for an alpha particle?
(a) 4He2
(b) 1n0
(c) 0e-1
(d) 1p1
Answer. (a)
2. Of the following, which is the most damaging when ingested?
(a) beta emitters
(b) alpha emitters
(c) gamma emitters
(d) all of these
Answer. (b)
3. An alpha particle is _______
(a) an electron
(b) one neutron and one proton
(c) two protons and two neutrons
(d) an X-ray emission
Answer. (c)
4. By what type of decay process might 214Pb convert to 214Bi?
(a) beta decay
(b) alpha decay
(c) gamma decay
(d) electron capture
Answer. (a)
5. The curie is a measure of the _______
(a) lethal threshold for radiation exposure
(b) number of alpha particles emitted by exactly 1 g of a radioactive substance
(c) number of disintegrations per second of a radioactive substance
(d) total energy absorbed by an object exposed to a radioactive source
Answer. (c)
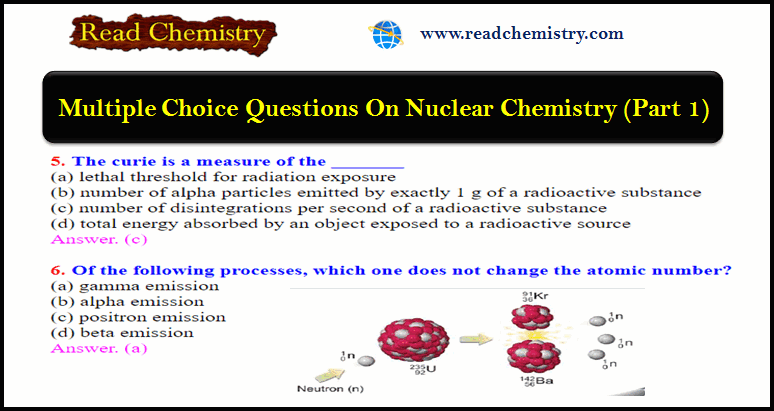
6. Of the following processes, which one does not change the atomic number?
(a) gamma emission
(b) alpha emission
(c) positron emission
(d) beta emission
Answer. (a)
7. Which of these nuclides is most likely to be radioactive?
(a) 39K19
(b) 27Al13
(c) 127I53
(d) 243Am95
Answer. (d)
8. The beta particle consists of _______
(a) high-energy rays
(b) 1 neutron
(c) 2 neutrons and 2 protons
(d) 1 electron
Answer. (d)
9. Lab coats and gloves provide shielding from _______
(a) alpha radiation
(b) alpha and beta radiation
(c) alpha, beta, and gamma radiation
(d) gamma radiation
Answer. (b)
10. When an alpha particle is released in nuclear decay, the mass number of the nucleus undergoing decay _______
(a) stays the same
(b) increases by 4
(c) decreases by 4
(d) decreases by 2
Answer. (c)
11. Which one of the following processes could not result in the conversion of strontium to rubidium?
(a) gamma emission
(b) proton emission
(c) electron capture
(d) positron emission
Answer. (a)
12. Alpha decay of 226Ra will yield which of the following nuclides?
(a) 222Rn
(b) 230Th
(c) 222Fr
(d) 222Th
Answer. (a)
13. In a Geiger-Müller counter, one “count” is directly due to _______
(a) a secondary electron
(b) a primary electron
(c) many electrons and ions
(d) a beta particle
Answer. (c)
14. 8Li3 decays to 8Be4. What type of decay is this?
(a) positron emission
(b) beta
(c) γ-ray
(d) alpha
Answer. (b)
15. _______ rays consist of He nuclei, while _______ rays are electromagnetic radiation.
(a) beta, alpha
(b) alpha, beta
(c) alpha, gamma
(d) gamma, beta
Answer. (c)
16. Which type of radioactive decay results in an increase in atomic number?
(a) positron emission
(b) alpha emission
(c) gamma emission
(d) beta emission
Answer. (d)
17. When 249Cf is bombarded with 10B, 257Lr is formed. What other particle(s) is/are produced?
(a) −1e0
(b) 1n0
(c) 0e1
(d) 1p1
Answer. (b)
18. Which combination of the number of protons and the number of neutrons is most common among the _______ naturally occurring non-radioactive nuclides?
(a) even protons; even neutrons
(b) odd protons; even neutrons
(c) even protons; odd neutrons
(d) odd protons; odd neutrons
Answer. (a)
19. Which form of radioactivity is most penetrating?
(a) alpha particles
(b) beta particles
(c) neutrons
(d) gamma rays
Answer. (d)
20. In electron capture, _______
(a) gamma rays are emitted
(b) a neutron is formed
(c) a positron is formed
(d) an alpha particle is emitted
Answer. (b)
21. The spontaneous transformation of one nuclide into others occurs only if _______
(a) the process is endothermic
(b) the process results in a neutron/proton ratio of 1:0 in the products
(c) sufficient energy can be absorbed from the surroundings to drive the process
(d) the combined mass of the products is less than the mass of the original nuclide
Answer. (d)
22. What particle is released when Ga-75 decays to Ge-75?
(a) neutron
(b) beta
(c) gamma
(d) alpha
Answer. (b)
23. What particle is missing in the following bombardment reaction? Al27 + ? = 1n + P30
(a) neutron
(b) beta
(c) proton
(d) alpha
Answer. (d)
24. What would be the immediate product of neutron absorption by 107Ag?
(a) 107Pd
(b) 109In
(c) 108Cd
(d) 108Ag
Answer. (d)
25. When a nuclide undergoes beta decay,_______
(a) the atomic number remains unchanged and the mass number increases by one
(b) the mass number remains unchanged and the atomic number decreases by one
(c) the mass number remains unchanged and the atomic number increases by one
(d) the atomic number remains unchanged and the mass number decreases by one
Answer. (c)
26. The half-life of 32P, which is used as a label on red blood cells to determine blood volume, is 14.3 days. How many days are required for the activity of a sample of 32P to drop to 5.00% of its initial level?
(a) 26.8 days
(b) 42.8 days
(c) 61.8 days
(d) 0.209 days
27. The half-life of Sulfur-35 is 88 days. If 8.0 g of Sulfur-35 exists on day one, what fraction will remain after 264 days?
(a) 0.5 g
(b) 4.0 g
(c) 0 g
(d) 1.0 g
Answer. (d)
28. When Xenon-123 emits a gamma ray, what is the product?
(a) 123Xe 54
(b) 123I53
(c) 119Te52
(d) 123Cs55
Answer. (a)
29. The cloth shroud from around a mummy is found to have a 14C activity of 8.9 disintegrations per minute per gram of carbon as compared with living organisms that undergo15.2 disintegrations per minute per gram of carbon. From the half-life for 14C decay, 5.73 × 103 yr, calculate the age of the shroud.
(a) 9.3 × 10–5 yr
(b) 4.4 × 103 yr
(c) 6.5 × 10–5 yr
(d) 1.92 × 103 yr
Answer. (b)
30.198Au has a half-life of 2.70 days. Assuming you start with a 10.0 mg sample of 198Au, how much will remain after 10.0 days?
(a) 0.246 mg
(b) 130 mg
(c) 0.768 mg
(d) 9.44 mg
Answer. (c)
31. The half-life for the beta decay of 233Pa is 27.4 days. How many days must pass to reduce a 5.00 g sample of 233Pa to 0.625 g?
(a) 109.6 days
(b) 54.8 days
(c) 82.2 days
(d) 27.4 days
Answer. (c)
32. The half-life of tritium (Hydrogen-3) is 12.3 yr. If 48.0 mg of tritium is released from a nuclear power plant during the course of an accident, what mass of this nuclide will remain after 49.2 yr?
(a) 6.0 mg
(b) 3.0 mg
(c) 24.0 mg
(d) 12.0 mg
Answer. (b)
33. How old is a fossil bone whose 14C content is 15.0 percent that of living bone?
(a) 25400 yr
(b) 15600 yr
(c) 380 yr
(d) 6810 yr
Answer. (b)
34. Iodine-131 has a half-life of 8 days. How many grams of I-131 in a 4.0 g sample remain after 24 days?
(a) 2.0 g
(b) 1.0 g
(c) 0.50 g
(d) 0.0 g
Answer. (c)
35. The half-life of 45Ca is 165 days. After 1.0 year, what percentage of the original sample of 45Ca remains?
(a) 10.9 percent
(b) 99.6 percent
(c) 2.16 percent
(d) 21.6 percent
Answer. (d)
36. What particle is produced when Phosphorus-29 decays to silicon-29?
(a) positron
(b) beta
(c) gamma
(d) alpha
Answer. (a)
37. The bombardment of which isotope by a neutron produces 198Au and proton?
(a) 199Tl81
(b) 197Hg80
(c) 198Hg80
(d) 197Pt78
Answer. (c)
38. Complete and balance the following nuclear equation by selecting the missing particle
24Mg12+ 2H1 → 4He2 + ?
(a) 22Al13
(b) 22Na11
(c) 26Al13
(d) 20Ne10
Answer. (b)
39. What is the product of the beta decay of 159Gd64?
(a) 159Tb65
(b) 159Eu63
(c) 159Gd64
(d) 155Sm62
Answer. (a)
40. Which of the following is the nuclear equation for bismuth-214 undergoing beta decay?
(a) 214Bi83→ 0e1+ 214Po84
(b) 214Bi83→ 0e1 + 214Pb82
(c) 214Bi83→ 0e1+ 214Po84
(d) 214Bi83→ 0e1+ 214Po84
Answer. (a)
Reference: Nuclear Chemistry chapter- Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition.