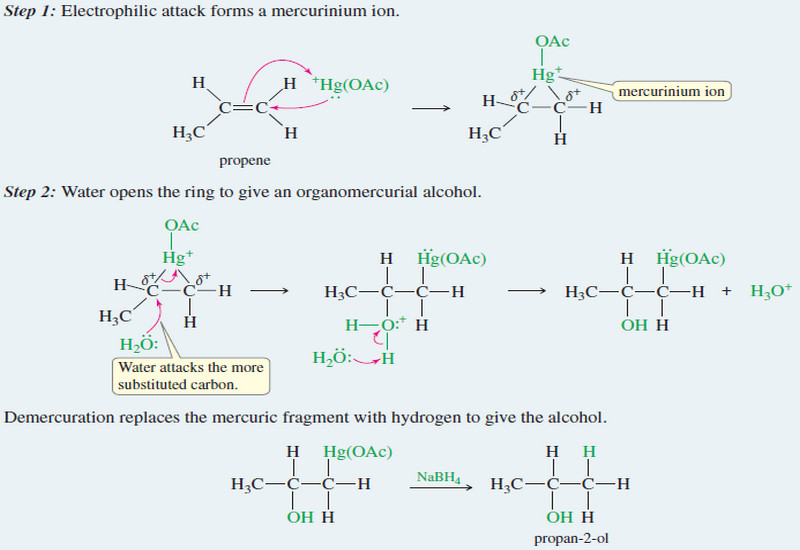
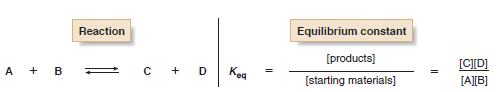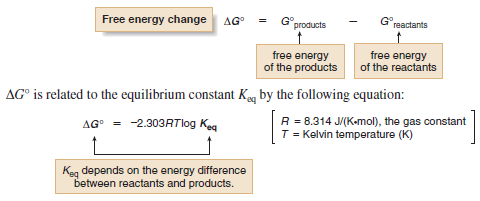Thermodynamics of Organic Compounds
– In this subject, we will discuss the Thermodynamics of Organic Compounds
Thermodynamics of Organic Compounds
– For a reaction to be practical, the equilibrium must favor the products, and the reaction rate must be fast enough to form them in a reasonable time.
– These two conditions depend on the thermodynamics and the kinetics of a reaction, respectively.
– Thermodynamics describes energy and equilibrium. How do the energies of the reactants and the products compare? What are the relative amounts of reactants and products at equilibrium?
– Kinetics describes reaction rates. How fast are reactants converted to products?
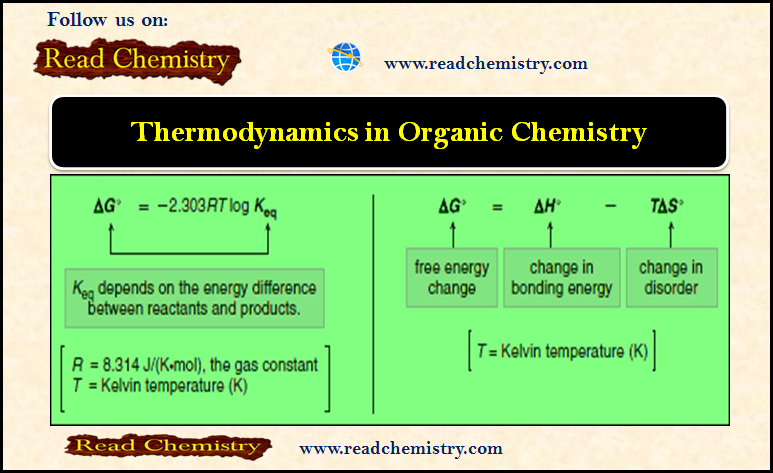
Equilibrium Constant and Free Energy Changes, ΔG°
– The equilibrium constant, Keq, is a mathematical expression that relates the amount of starting material and product at equilibrium.
– For example, when starting materials A and B react to form products C and D, the equilibrium constant is given by the following expression:
– The size of Keq tells about the position of equilibrium; that is, it expresses whether the starting materials or products predominate once equilibrium has been reached.
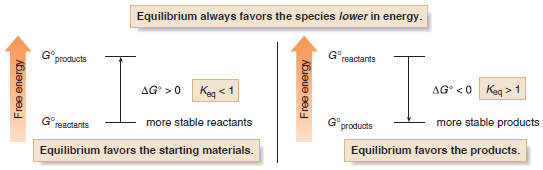
(1) When Keq > 1, equilibrium favors the products (C and D) and the equilibrium lies to the right as the equation is written.
(2) When Keq < 1, equilibrium favors the starting materials (A and B) and the equilibrium lies to the left as the equation is written.
– For a reaction to be useful, the equilibrium must favor the products, and Keq > 1.
– What determines whether equilibrium favors the products in a given reaction?
– The position of equilibrium is determined by the relative energies of the reactants and products.
– The free energy of a molecule, also called its Gibbs free energy, is symbolized by G°.
– The change in free energy between reactants and products, symbolized by ΔG°, determines whether the starting materials or products are favored at equilibrium.
– ΔG°, Gibbs free energy, is the overall energy difference between reactants and products.
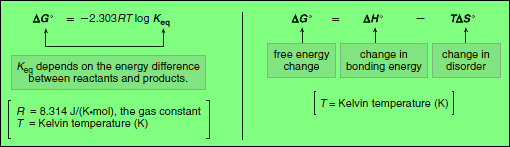
– Using this expression we can determine the relationship between the equilibrium constant and the free energy change between reactants and products.
(1) When Keq > 1, log Keq is positive, making ΔG° negative, and energy is released.
– Thus, equilibrium favors the products when the energy of the products is lower than the energy of the reactants.
(2) When Keq < 1, log Keq is negative, making ΔG° positive, and energy is absorbed.
– Thus, equilibrium favors the reactants when the energy of the products is higher than the energy of the reactants.
– Compounds that are lower in energy have increased stability.
– Thus, equilibrium favors the products when they are more stable (lower in energy) than the starting materials of a reaction.
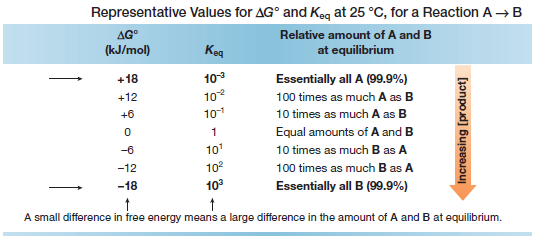
– Because ΔG° depends on the logarithm of Keq, a small change in energy corresponds to a large difference in the relative amount of starting material and product at equilibrium.
– Several values of ΔG° and Keq are given in the following table:
– For example, a difference in energy of only ~6 kJ/mol means that there is 10 times as much of the more stable species at equilibrium.
– A difference in energy of ~18 kJ/mol means that there is essentially only one compound, either starting material or product, at equilibrium.
Conclusion: Thermodynamics of Organic Compounds
– Conditions Favoring Product Formation:
Energy Changes and Conformational Analysis
– These equations can be used for any process with two states in equilibrium.
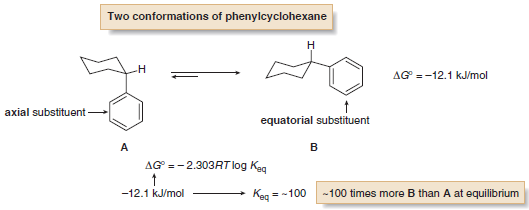
– As an example, monosubstituted cyclohexanes exist as two different chair conformations that rapidly interconvert at room temperature, with the conformation having the substituent in the roomier equatorial position favored.
– Knowing the energy difference between the two conformations allows us to calculate the amount of each at equilibrium.
– For example, the energy difference between the two chair conformations of phenylcyclohexane is –12.1 kJ/mol, as shown in the accompanying equation.
– Using the values in the table above, this corresponds to an equilibrium constant of ~100, meaning that there is approximately 100 times more B (equatorial phenyl group) than A (axial phenyl group) at equilibrium.
Thermodynamics of Organic Compounds: Enthalpy and Entropy
– The free energy change (ΔG°) depends on the enthalpy change (ΔH°) and the entropy change (ΔS°).
– ΔH° indicates relative bond strength, but what does ΔS° measure?
– Entropy (S°) is a measure of the randomness in a system.
– The more freedom of motion or the more disorder present, the higher the entropy.
– Gas molecules move more freely than liquid molecules and are higher in entropy.
– Cyclic molecules have more restricted bond rotation than similar acyclic molecules and are lower in entropy.
– The entropy change (ΔS°) is the change in the amount of disorder between reactants and products.
– ΔS° is positive (+) when the products are more disordered than the reactants.
– ΔS° is negative (–) when the products are less disordered (more ordered) than the reactants.
– Reactions resulting in an increase in entropy are favored.
The relationship between ΔG° is related to ΔH°
– ΔG° is related to ΔH° and ΔS° by the following equation:
This equation tells us that the total energy change in a reaction is due to two factors:
(A) The change in the bonding energy
– The change in bonding energy can be calculated from bond dissociation energies
(B) The change in disorder (Entropy).
– Entropy changes are more difficult to access, but they are important in the following two cases:
(1) When the number of molecules of starting material differs from the number of molecules of product in the balanced chemical equation.
(2) When an acyclic molecule is cyclized to a cyclic one, or a cyclic molecule is converted to an acyclic one.
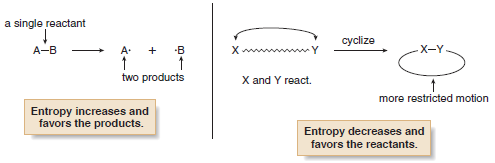
– For example, when a single starting material forms two products, as in the homolytic cleavage of a bond to form two radicals, entropy increases and favors formation of the products.
– Entropy decreases when an acyclic compound forms a ring because a ring has fewer degrees of freedom.
– In this case, therefore, entropy does not favor the formation of the product.
– In most other reactions that are not carried out at high temperatures, the entropy term (TΔS°) is small compared to the enthalpy term (ΔH°) and it can be neglected.
– Thus, we will often approximate the overall free energy change of a reaction by the change in the bonding energy only.
– Keep in mind that this is an approximation, but it gives us a starting point from which to decide if the reaction is energetically favorable.
– According to this approximation:
(1) The product is favored in reactions in which ΔH° is a negative value;
– that is, the bonds in the product are stronger than the bonds in the starting material.
(2) The starting material is favored in a reaction in which ΔH° is a positive value;
– that is, the bonds in the starting material are stronger than the bonds in the product.
Reference: Organic chemistry / T.W. Graham Solomons, Craig B.Fryhle, Scott A. Snyder, / ( eleventh edition) / 2014.