Subatomic Particles: Electrons, Protons, and Neutrons
– In this subject, we will discuss the Subatomic Particles: Electrons, Protons, and Neutrons
Subatomic Particles
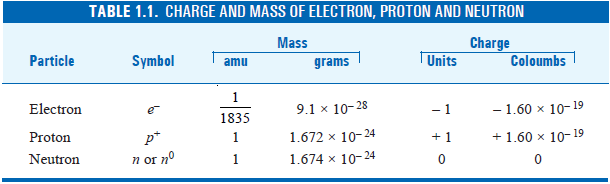
– We have hitherto studied the properties of the three principal fundamental particles of the atom, namely the electron, proton, and neutron.
– These are summarised in this Table:
– Nearly all of the ordinary chemical properties of matter can be examined in terms of atoms consisting of electrons, protons, and neutrons.
– Therefore for our discussion, we will assume that the atom contains only these three principal subatomic particles.
(1) The Electrons
– The knowledge about the electron was derived as a result of the study of the electric discharge in the discharge tube (J.J. Thomson, 1896).
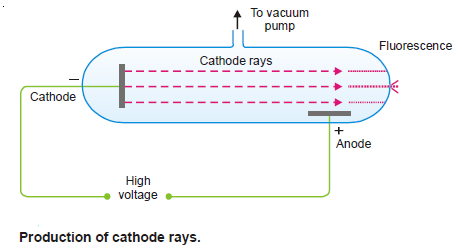
(1) The discharge tube consists of a glass tube with metal electrodes fused in the walls.
(2) Through a glass side-arm air can be drawn with a pump.
(3) The electrodes are connected to a source of high voltage (10,000 Volts) and the air partially evacuated.
(4) The electric discharge passes between the electrodes and the residual gas in the tube begins to glow.
– If virtually all the gas is evacuated from within the tube, the glow is replaced by faintly luminous ‘rays’ which produce fluorescence on the glass at the end far from the cathode.
– The rays that proceed from the cathode and move away from it at right angles in straight lines are called Cathode Rays.
Properties of Cathode Rays
(1) They travel in straight lines away from the cathode and cast shadows of metallic objects placed in their path.
(2) Cathode rays cause the mechanical motion of a small pin-wheel placed in their path. Thus they possess kinetic energy and must be material particles.
(3) They produce fluorescence (a glow) when they strike the glass wall of the discharge tube.
(4) They heat up a metal foil to incandescence which they impinge upon.
(5) Cathode rays produce X-rays when they strike a metallic target.
(6) Cathode rays are deflected by the electric as well as the magnetic field in a way indicating that they are streams of minute particles carrying negative charge.
– By counterbalancing the effect of magnetic and electric field on cathode rays.
– Thomson was able to work out the ratio of the charge and mass (e/m) of the cathode particle.
– In SI units the value of (e/m) of cathode particles is – 1.76 × 188 coulombs per gram.
– As a result of several experiments, Thomson showed that the value of e/m of the cathode particle was the same regardless of both the gas and the metal of which the cathode was made.
– This proved that the particles making up the cathode rays were all identical and were constituent parts of the various atoms.
– Dutch Physicist H.A. Lorentz named them Electrons.
– Electrons are also obtained by the action of X-rays or ultraviolet light on metals and from heated filaments.
– These are also emitted as β-particles by radioactive substances.
– Thus it is concluded that electrons are a universal constituent of all atoms.
Definition of an electron
– Having known the charge and mass of an electron, it can be defined as :
(1) An electron is a subatomic particle which bears charge – 1.60 × 10–19 coulomb and has mass 9.1 × 10–28 g.
(2) An electron is A particle that bears one unit negative charge and mass 1/1835th of a hydrogen atom.
Since an electron has the smallest charge known, it was designated as a unit charge by Thomson.
Positive Rays
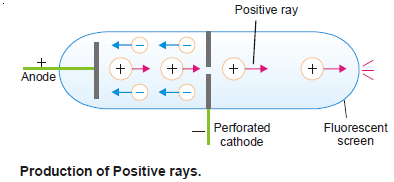
– In 1886 Eugen Goldstein used a discharge tube with a hole in the cathode.
– He observed that while cathode rays were streaming away from the cathode, there were colored rays produced simultaneously which passed through the perforated cathode and caused a glow on the wall opposite to the anode.
– Thomson studied these rays and showed that they consisted of particles carrying a positive charge. He called them Positive rays.
Properties of Positive Rays
(1) They travel in a straight line in a direction opposite to the cathode.
(2) They are deflected by electric as well as magnetic field in a way indicating that they are positively charged.
(3) The charge-to-mass ratio (e/m) of positive particles varies with the nature of the gas placed in the discharge tube.
(4) They possess mass many times the mass of an electron.
(5) They cause fluorescence in zinc sulfide.
How are Positive rays produced?
– When high-speed electrons (cathode rays) strike molecule of a gas placed in the discharge tube, they knock out one or more electrons from it.
– Thus a positive ion results:
– These positive ions pass through the perforated cathode and appear as positive rays.
– When an electric discharge is passed through the gas under high electric pressure, its molecules are dissociated into atoms and the positive atoms (ions) constitute the positive rays.
Conclusions from the study of Positive rays
– From a study of the properties of positive rays, Thomson and Aston (1913) concluded that atom consists of at least two parts:
(a) the electrons
(b) a positive residue with which the mass of the atom is associated.
(2) The Protons
– E. Goldstein (1886) discovered protons in the discharge tube containing hydrogen.
– It was J.J. Thomson who studied their nature.
– He showed that :
(1) The actual mass of proton is 1.672 × 10–24 gram. On the relative scale, proton has mass 1 atomic mass unit (amu).
(2) The electrical charge of proton is equal in magnitude but opposite to that of the electron.
– Thus proton carries a charge +1.60 × 10–19coulombs or + 1 elementary charge unit.
– Since proton was the lightest positive particle found in atomic beams in the discharge tube, it was thought to be a unit present in all other atoms.
– Protons were also obtained in a variety of nuclear reactions indicating further that all atoms contain protons.
– Thus A proton is defined as a subatomic particle that has a mass of 1 amu and charge + 1 elementary charge unit.
– A proton is a subatomic particle which has one unit mass and one unit positive charge.
(3) The Neutrons
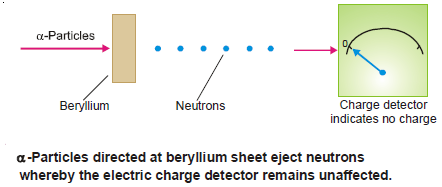
– In 1932 Sir James Chadwick discovered the third subatomic particle.
– He directed a stream of alpha particles 4He2 at a beryllium target.
– He found that a new particle was ejected.
– It has almost the same mass (1.674 × 10–24g) as that of a proton and has no charge.
– He named it neutron.
– The assigned relative mass of a neutron is approximately one atomic mass unit (amu).
– Thus :
– A neutron is a subatomic particle that has a mass almost equal to that of a proton and has no charge.
– The reaction that occurred in Chadwick’s experiment is an example of artificial transmutation where an atom of beryllium is converted to a carbon atom through the nuclear reaction.
(4) Other Subatomic Particles
– Besides electrons, protons, and neutrons, many other subatomic particles such as mesons, positrons, neutrinos, and antiprotons have been discovered.
– A great deal of recent research is producing a long list of still other subatomic particles with names quarks, pions, and gluons.
– With each discovery, the picture of atomic structure becomes increasingly complex.
– Fortunately, the three-particle (electron, proton, neutron) picture of the atom still meets the needs of the chemists.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.