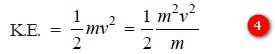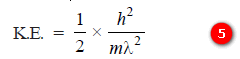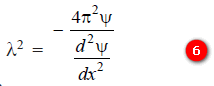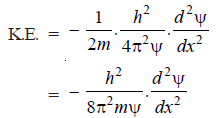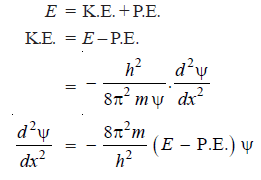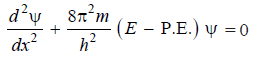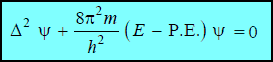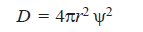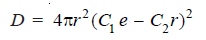Schrödinger Wave Equation
Schrödinger Wave Equation
– In order to provide sense and meaning to the probability approach, Schrödinger derived an equation known after his name as the Schrödinger Wave Equation.
– Calculation of the probability of finding the electron at various points in an atom was the main problem before Schrödinger.
– His equation is the keynote of wave mechanics and is based upon the idea of the electron as (standing wave) around the nucleus.
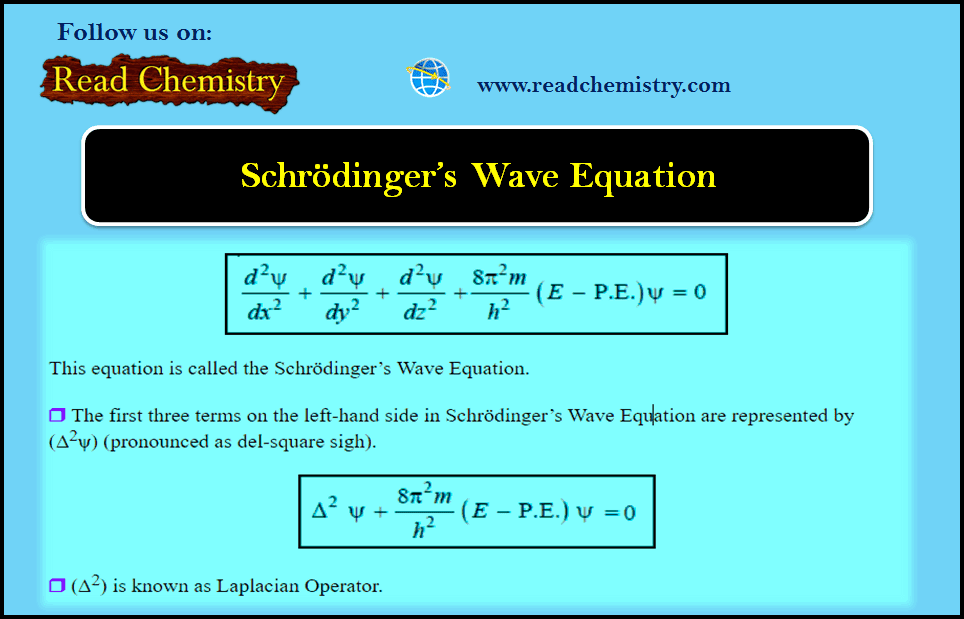
– The equation for the standing wave, comparable with that of a stretched string is:
– where ψ (pronounced as sigh) is a mathematical function representing the amplitude of wave (called wave function) (x), the displacement in a given direction, and (λ), the wavelength, and (A) is a constant.
– By differentiating equation (a) twice with respect to (x), we get:
– The K.E. of the particle of mass m and velocity (ν) is given by the relation:
– According to Broglie’s equation:
– Substituting the value of (m2 v2), we have:
– From equation (3), we have:
– Substituting the value of (λ2) in equation (5):
– The total energy E of a particle is the sum of kinetic energy and the potential energy:
– This is Schrödinger’s equation in one dimension. It need be generalised for a particle whose motion is described by three space coordinates x, y and z. Thus,
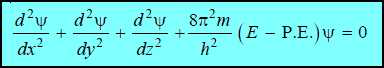
– This equation is called the Schrödinger Wave Equation.
– The first three terms on the left-hand side in Schrödinger Wave Equation are represented by (Δ2ψ) (pronounced as del-square sigh).
(Δ2) is known as Laplacian Operator.
– The Schrödinger wave equation is a second degree differential equation.
– It has several solutions. Some of these are imaginary and are not valid.
– If the potential energy term is known, the total energy (E) and the corresponding wave function (ψ) can be evaluated.
– The wave function is always finite, single valued and continuous. It is zero at infinite distance.
– Solutions that meet these requirements are only possible if (E) is given certain characteristic values called Eigen-values.
– Corresponding to these values of (E), we have several characteristic values of wave function (ψ) and are called Eigen-functions.
– As the Eigen-values correspond very nearly to the energy values associated with different Bohr-orbits, the Bohr’s model may be considered as a direct consequence of wave mechanical approach.
Significance of ψ and ψ2
– In Schrödinger wave equation (ψ) represents the amplitude of the spherical wave.
– According to the theory of propagation of light and sound waves, the square of the amplitude of the wave is proportional to the intensity of the sound or light.
– A similar concept, modified to meet the requirement of uncertainty principle, has been developed for the physical interpretation of wave function(ψ).
– This may be stated as the probability of finding an electron in an extremely small volume around a point.
– It is proportional to the square of the function (ψ2) at that point.
– If wave function (ψ) is imaginary, (ψψ*) becomes a real quantity where (ψ*) is a complex conjugate of (ψ).
– This quantity represents the probability (ψ2) as a function of x, y, and z coordinates of the system, and it varies from one space region to another.
– Thus the probability of finding the electron in different regions is different.
– This is in agreement with the uncertainty principle and gave a death blow to Bohr’s concept.
– In Schrödinger Wave Equation, the symbol (ψ) represents the amplitude of the spherical wave.
– For hydrogen atom, Schrödinger’s Wave Equation gives the wave function of the electron (with energy = – 2.18 × 10–11ergs) situated at a distance (r),

where C1 and C2 are constants.
– The square of the amplitude( ψ2) is proportional to the density of the wave.
– The wave of energy or the cloud of negative charge is denser in some parts than in others.
– Max Born interpreted the wave equations on the basis of probabilities.
– Even if an electron be considered as a particle in motion around the nucleus, the wave equation may be interpreted in terms of probability or relative chance of finding the electron at any given distance from the nucleus.
– The space characteristic of an electron is best described in terms of the distribution function given by:
– The numerical value of (D) denotes the probability or chance of finding the electron in a shell of radius (r) and thickness (dr), or of volume (4πr2dr).
– Substituting for (ψ) we have:
– The probability of finding the electron is clearly a function of (r).
– When r = 0 or ∝ , the probability function (D) becomes equal to zero.
– In other words, there is no probability of finding the electron at the nucleus or at infinity.
– However, it is possible to choose a value of (r) such that there is 90-95 percent chance of finding the electron at this distance.
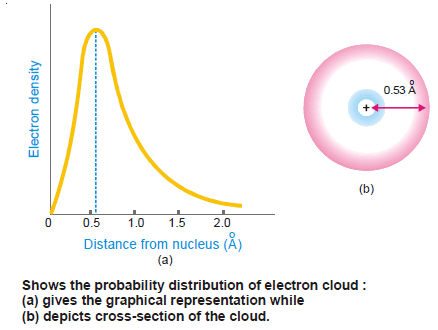
– For the hydrogen atom, this distance is equal to 0.53 × 10–8cm or 0.53 Å.
– If the probability distribution be plotted against the distance r from the nucleus, the curve obtained is shown in Fig (1).
– The probability distribution is maximum at the distance 0.53 Å and spherically symmetrical.
– This distance corresponds to Bohr’s first radius (a0).
– The graph can be interpreted as representing a contour that encloses a high percentage of charge.
– When the electron gets excited and it is raised from n to higher energy levels (say n = 2 or n = 3), the solution of wave equation gives sets of value of (ψ2) which give different shapes to the space distribution of the electron.
Charge Cloud Concept and Orbitals
– The Charge Cloud Concept finds its birth from wave mechanical theory of the atom.
– The wave equation for a given electron, on solving gives a three-dimensional arrangement of points where it can possibly lie.
– There are regions where the chances of finding the electron are relatively greater.
– Such regions are expressed in terms of (cloud of negative charge).
– We need not know the specific location of the electrons in space but are concerned with the negative charge density regions.
– Electrons in atoms are assumed to be vibrating in space, moving haphazardly but at the same time are constrained to lie in regions of highest probability for most of the time.
– The charge cloud concept simply describes the high probability region.
– The three-dimensional region within which there is higher probability that an electron having a certain energy will be found is called an orbital.
– An orbital: is the most probable space in which the electron spends most of its time while in constant motion.
– In other words, it is the spatial description of the motion of an electron corresponding to a particular energy level.
– The energy of an electron in an atomic orbital is always the same.
– Each energy level corresponds to a three-dimensional electron wave which envelopes the nucleus.
– This wave possesses a definite ‘size’, ‘shape’, and ‘orientation’ and thus can be represented pictorially.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.