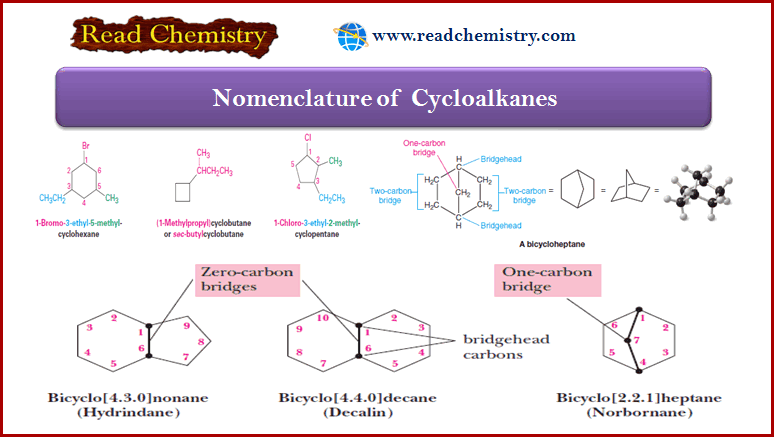
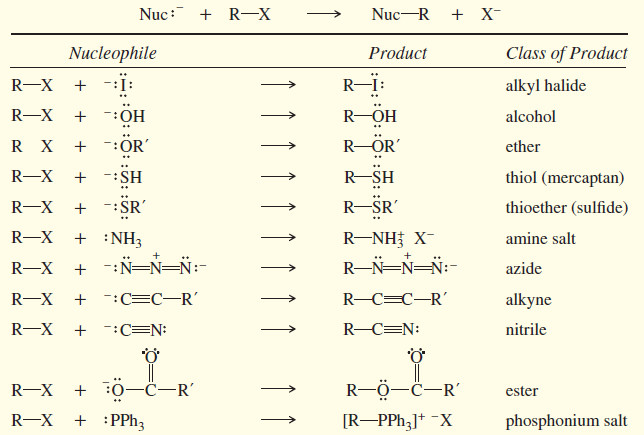
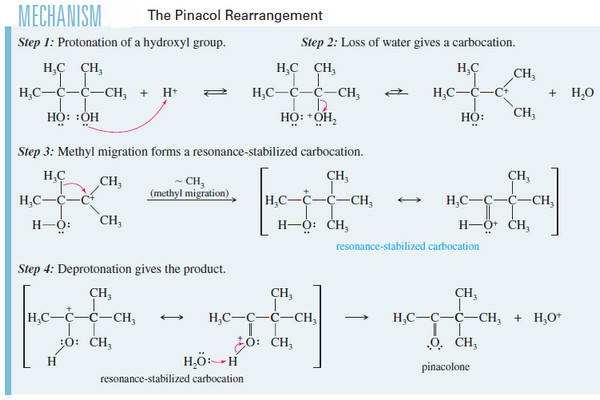
Biological Discrimination of Enantiomers
Biological Discrimination of Enantiomers
– If the direction of rotation of polarized light were the only difference between enantiomers, one might ask whether the difference would be important.
– Biological systems commonly distinguish between enantiomers, and two enantiomers may have totally different biological properties.
– In fact, any chiral probe can distinguish between enantiomers, and a polarimeter is only one example of a chiral probe.
– Another example is your hand. If you needed to sort a box of gloves into right-handed gloves and left-handed gloves, you could distinguish between them by checking to see which ones fit your right hand.
– Enzymes in living systems are chiral, and they are capable of distinguishing between enantiomerrs.
– Usually, only one enantiomer of a pair fits properly into the chiral active site of an enzyme.
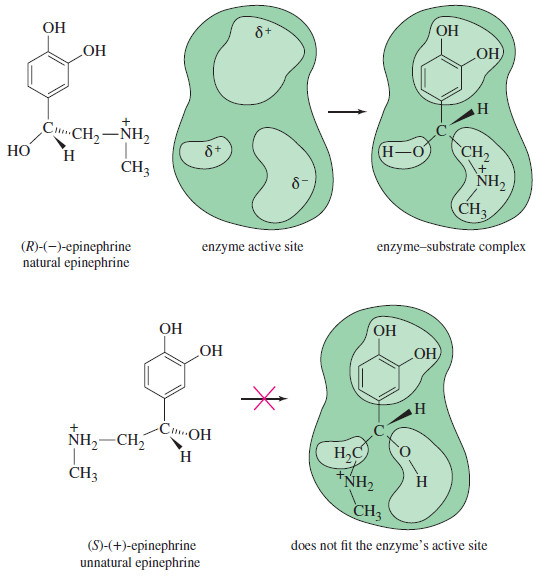
– For example, the levorotatory form of epinephrine is one of the principal hormones secreted by the adrenal medulla.
– When synthetic epinephrine is given to a patient, the form has the same stimulating effect as the natural hormone.
– The form lacks this effect and is mildly toxic.
– The following Figure shows a simplified picture of how only the enantiomer fits into the enzyme’s active site.
– Biological systems are capable of distinguishing between the enantiomers of many different chiral compounds.
– In general, just one of the enantiomerrs produces the characteristic effect; the other either produces no effect or has a different effect.
– Even your nose is capable of distinguishing between some enantiomers. For example, is the fragrance associated with spearmint oil; has the tangy odor of caraway seed.
– The receptor sites for the sense of smell must be chiral, therefore, just as the active sites in most enzymes are chiral.
– In general, enantiomerrs do not interact identically with other chiral molecules, whether or not they are of biological origin
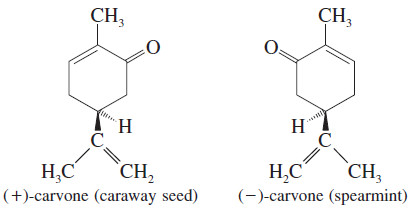
Application: Enantiomers in Biochemistry
– Enzymes can exist as two enantiomers, although only one enantiomer is found in nature.
– In 1992, Stephen Kent and co-workers reported the synthesis of both enantiomers of an enzyme that cuts peptide substrates, and showed for the first time that each enzyme acts only on the corresponding enantiomeric peptide substrate.