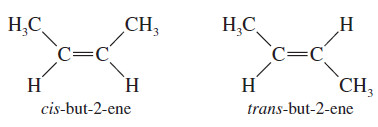Physical Properties of Diastereomers
What is Diastereomers?
– We have defined stereoisomers as isomers whose atoms are bonded together in the same order but differ in how the atoms are directed in space.
– We have also considered enantiomers (mirror-image isomers) in detail.
– All other stereoisomers are classified as diastereomers, which are defined as stereoisomers that are not mirror images.
– Most diastereomers are either geometric isomers or compounds containing two or more chirality centers.
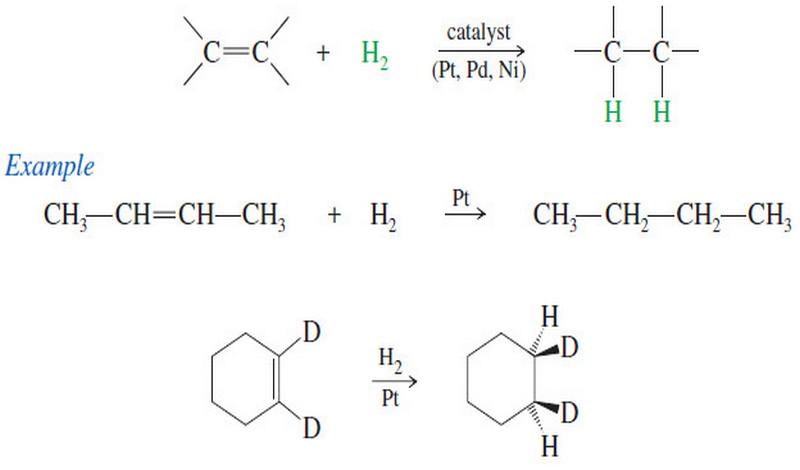
Cis-trans Isomerism on Double Bonds
– We have already seen one class of diastereomers, the cis-trans isomers, or geometric isomers.
– For example, there are two isomers of but-2-ene:
– These stereoisomers are not mirror images of each other, so they are not enantiomers. They are diastereomers.
Cis-trans Isomerism on Rings
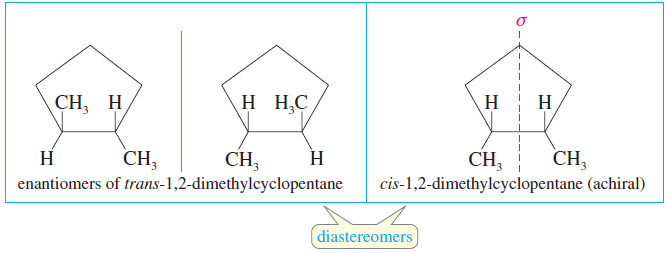
– Cis-trans isomerism is also possible when there is a ring present.
– Cis- and trans-1,2-dimethylcyclopentane are geometric isomers, and they are also diastereomerrs.
– The trans diastereomer has an enantiomer, but the cis diastereomer has an internal mirror plane of symmetry, so it is achiral.
Physical Properties of Diastereomers
– We have seen that enantiomers have identical physical properties except for the direction in which they rotate polarized light.
– Diastereomers, on the other hand, generally have different physical properties.
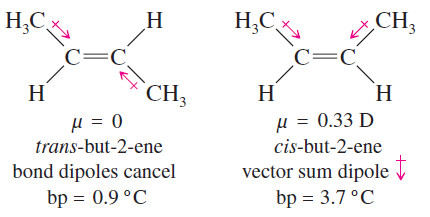
– For example, consider the diastereomerrs of but-2-ene (shown next).
– The symmetry of trans-but-2-ene causes the dipole moments of the bonds to cancel.
– The dipole moments in cis-but-2-ene do not cancel but add together to create a molecular dipole moment.
– The dipole–dipole attractions of cis-but-2-ene give it a higher boiling point than trans but-2-ene
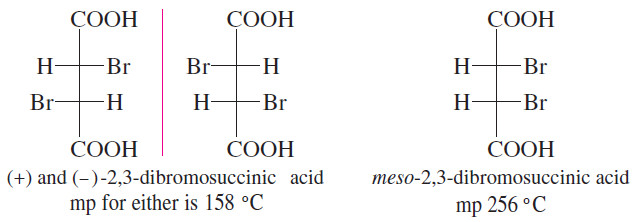
– Diastereomers that are not geometric isomers also have different physical properties.
– The two diastereomers of 2,3-dibromosuccinic acid have melting points that differ by nearly 100 °C!
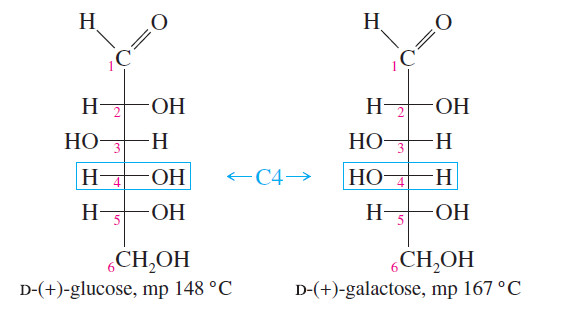
– Most of the common sugars are diastereomerrs of glucose.
– All these diastereomerrs have different physical properties.
– For example, glucose and galactose are diastereomeric sugars that differ only in the stereochemistry of one asymmetric carbon atom, C4.
– Because diastereomerrs have different physical properties, we can separate them by ordinary means such as distillation, recrystallization, and chromatography.
– As we will see in the next section, the separation of enantiomers is a more difficult process.