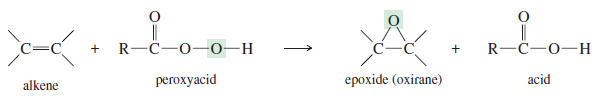Epoxidation of Alkenes
Epoxidation of Alkenes
– Some of the most important reactions of alkenes involve oxidation.
– When we speak of oxidation, we usually mean reactions that form carbon–oxygen bonds. (Halogens are oxidizing agents, and the addition of a halogen molecule across a double bond is formally an oxidation as well.)
– Oxidations are particularly important because many common functional groups contain oxygen, and alkene oxidations are some of the best methods for introducing oxygen into organic molecules.
– We will consider methods for epoxidation, dihydroxylation, and oxidative cleavage of alkene double bonds.
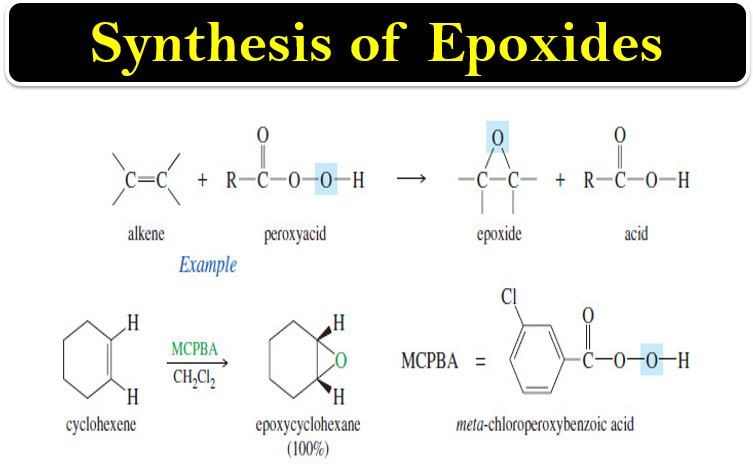
– An epoxide is a three-membered cyclic ether, also called an oxirane.
– Epoxides are valuable synthetic intermediates used for converting alkenes to a variety of other functional groups.
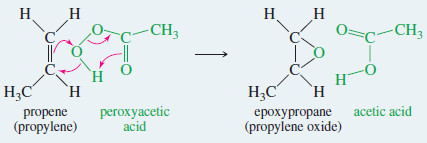
– An alkene is converted to an epoxide by a peroxyacid, a carboxylic acid that has an extra oxygen atom in a -O-O- (peroxy) linkage.
– The epoxidation of an alkene is clearly an oxidation, since an oxygen atom is added.
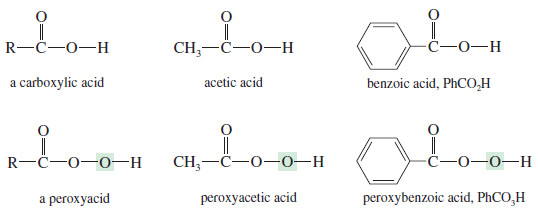
– Peroxyacids are highly selective oxidizing agents.
– Some simple peroxyacids (sometimes called peracids) and their corresponding carboxylic acids are shown next.
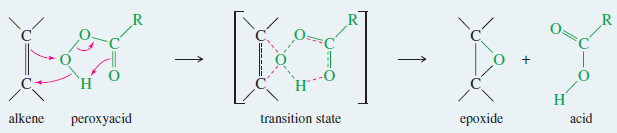
– A peroxyacid epoxidizes an alkene by a concerted electrophilic reaction where several bonds are broken and several are formed at the same time.
– Starting with the alkene and the peroxyacid, a one-step reaction gives the epoxide and the acid directly, without any intermediates
Mechanism: Epoxidation of Alkenes
Peroxyacids epoxidize alkenes in a one-step (concerted) process.
Example: Epoxidation of propene by peroxyacetic acid.
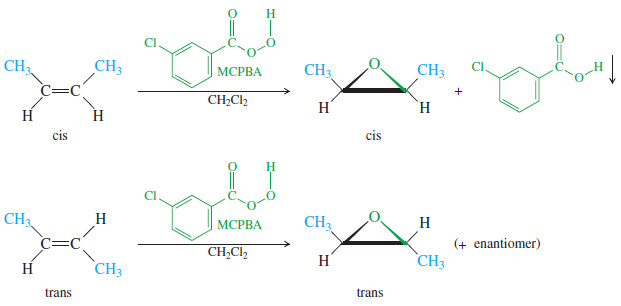
– Because the epoxidation takes place in one step, there is no opportunity for the alkene molecule to rotate and change its cis or trans geometry.
– The epoxide retains whatever stereochemistry is present in the alkene.
– The following examples use m-chloroperoxybenzoic acid (MCPBA), a common epoxidizing reagent, to convert alkenes to epoxides having the same cis or trans stereochemistry.
– MCPBA is used for its desirable solubility properties: The peroxyacid dissolves, then the spent acid precipitates out of solution
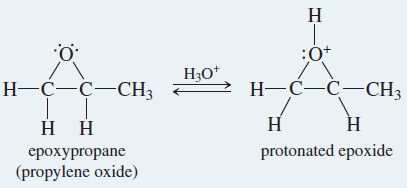
Acid-Catalyzed Opening of Epoxides
– Most epoxides are easily isolated as stable products if the solution is not too acidic.
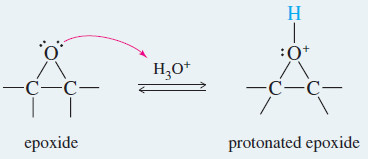
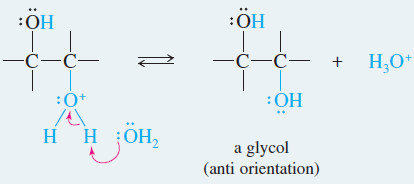
– Any moderately strong acid protonates the epoxide, however. Water attacks the protonated epoxide, opening the ring and forming a 1,2-diol, commonly called a glycol
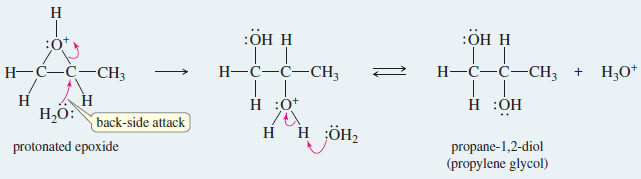
Mechanism: Acid-Catalyzed Opening of Epoxide
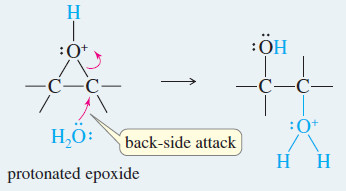
– The crucial step is a back-side attack by the solvent on the protonated epoxide.
Step 1: Protonation of the epoxide activates it toward nucleophilic attack
Step 2: Back-side attack by the solvent (water) opens the ring.
Step.3 : Deprotonation gives the diol product.
Example: Acid-catalyzed hydrolysis of propylene oxide (epoxypropane).
Step 1: Protonation of the epoxide
Steps 2 and 3: Back-side attack by water, then deprotonation of the product.
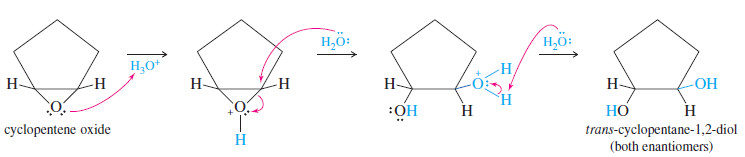
– Because glycol formation involves a back-side attack on a protonated epoxide, the result is anti orientation of the hydroxyl groups on the double bond.
– For example, when 1,2-epoxycyclopentane (cyclopentene oxide) is treated with dilute mineral acid, the product is pure trans-cyclopentane-1,2-diol.
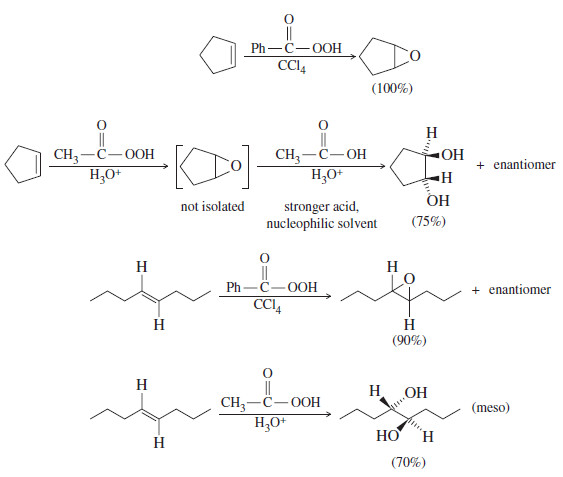
– Epoxidation reagents can be chosen to favor either the epoxide or the glycol. chiral diols.
– Peroxyacetic acid is used in strongly acidic water solutions.
– The acidic solution protonates the epoxide and converts it to the glycol. Peroxybenzoic acids are weak acids that can be used in nonnucleophilic solvents such as carbon tetrachloride.
m-Chloroperoxybenzoic acid in CCl4 generally gives good yields of epoxides.
– The following Figure compares the uses of these reagents.