Olefin Metathesis
Olefin Metathesis
– The double bond is the strongest bond in an alkene, yet it is also the most reactive bond.
– Imagine how useful it would be if we could break molecules at their double bonds and reassemble them as we please. That is the goal of olefin metathesis.
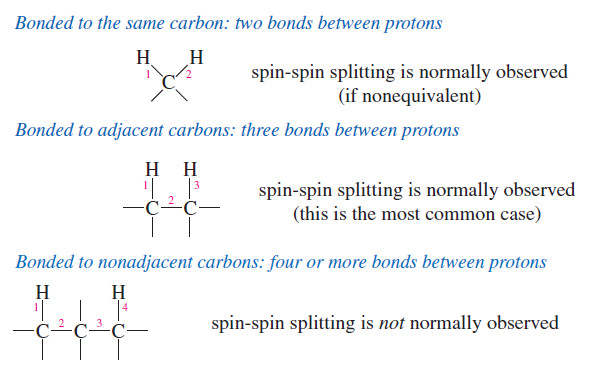
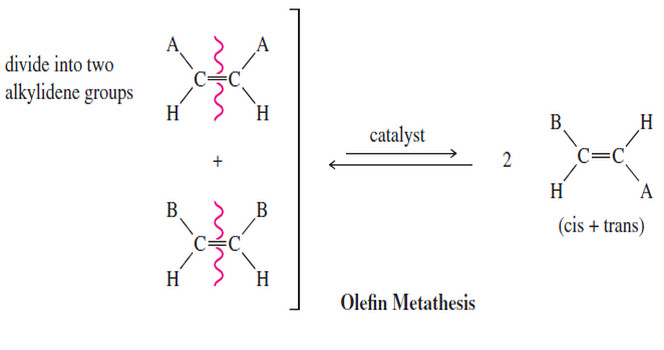
– We can think of an alkene as two alkylidene groups (=CHR) held together by the double bond, and mentally divide it up just like we divide the molecule when we go to name it as E or Z .
– Olefin metathesis is any reaction that trades and interchanges these alkylidene groups.
– The word metathesis comes from the Greek words meta (change) and thesis (position), meaning that the alkylidene groups change their positions in the products.
– The following figure shows the trading of alkylidene groups that takes place during olefin metathesis.
– The 2005 Nobel Prize in Chemistry was awarded to Yves Chauvin (French Petroleum Institute), Robert Grubbs (Caltech), and Richard Schrock (MIT) for developing effective ways to induce alkenes to undergo metathesis.
(1) Catalysts for Olefin Metathesis
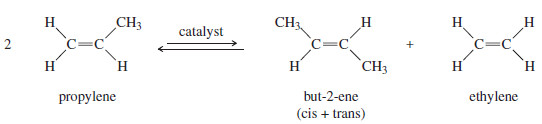
– Olefin metathesis was first observed in the 1950s, and was used in industry to convert propylene to a mixture of but-2-ene and ethylene.
– This Phillips Triolefin Process used an aluminum/molybdenum catalyst whose exact structure was unknown.
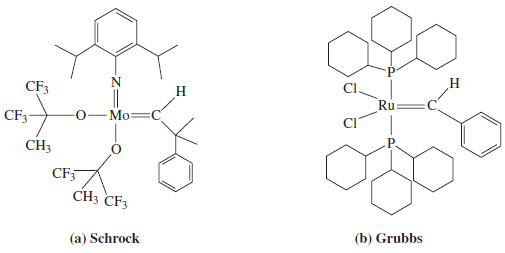
– Around 1990, Richard Schrock developed versatile molybdenum and tungsten catalysts for olefin metathesis that tolerate a wide range of functional groups in the alkylidene fragments of the olefins.
– The Schrock catalyst shown in Figure (a) is now commercially available.
– The Schrock catalysts tend to be air- and moisture-sensitive, which limits their use in commercial processes
– In 1992, Robert Grubbs developed a ruthenium phosphine catalyst (Figure b) that is less sensitive to oxygen and moisture than the Schrock catalysts, and tolerates even more functional groups in the alkylidene fragments of the olefins.
– Both the Schrock and Grubbs catalysts have a metal atom that is double-bonded to an alkylidene (=CHR) group.
– They can be symbolized [M]=CHR , where the [M] in brackets signifies that the metal atom has other ligands that fine-tune its reactivity.
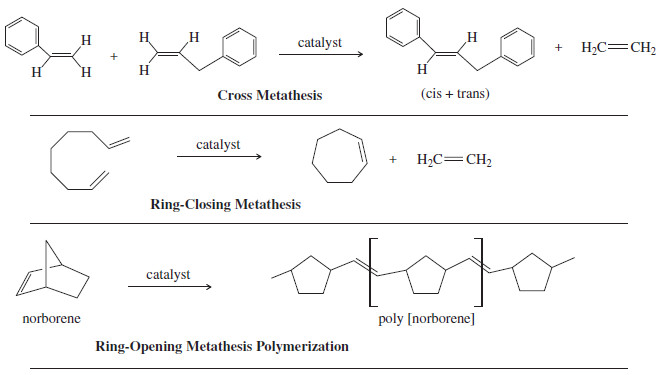
– The following figure shows some examples of useful reactions that are catalyzed by the Schrock and Grubbs catalysts.
– One important aspect of these metathesis reactions is that they are all reversible, so they form equilibrium mixtures of the reactants and all possible products unless something is done to drive the reaction toward the desired products.
– The first two examples in Figure above use the most common method, formation of ethylene gas.
– Ethylene bubbles off as it forms, effectively driving the reaction to completion.
– The ring-opening metathesis polymerization is exothermic and naturally goes to products because the ring strain in the bicyclic norbornene is released when the ring opens to form the polymer.
(2) Mechanism of Olefin Metathesis
– Several mechanisms were proposed to explain the catalytic metathesis reactions, but the mechanism published by Yves Chauvin in 1971 has come to be accepted as correct.
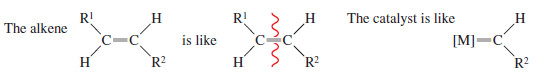
– We can think of an alkene as two alkylidene groups bonded together.
– Similarly, the Schrock and Grubbs catalysts are like a metal atom bonded to one alkylidene group.
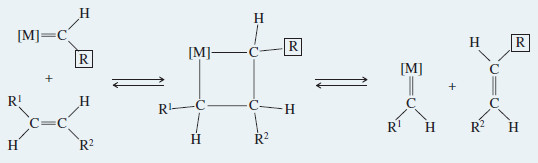
– Chauvin proposed that the metal-alkylidene catalyst forms an intermediate fourmembered ring with an alkene, as shown in the following Mechanism .
– Then the ring breaks apart, either to give the starting alkene and catalyst or to give a new alkene that has traded one alkylidene group with the catalyst.
– This mechanism allows the alkylidene groups to change partners back and forth with the catalytic metal until a thermodynamic equilibrium is reached.
– As we saw earlier, good yields of products result if there is an effective driving force (such as formation of a gaseous by-product or release of ring strain) to push the equilibrium toward the desired products.
Mechanism: Olefin Metathesis