Kinetic Properties of Sols – Brownian movement
Kinetic Properties of Sols
– In this topic, we will discuss Kinetic Properties of Sols.
Brownian Movement
– When a sol is examined with an ultramicroscope, the suspended particles are seen as shining specks of light.
– By following an individual particle it is observed that the particle is undergoing a constant rapid motion.
– It moves in a series of short straight-line paths in the medium, changing directions abruptly.
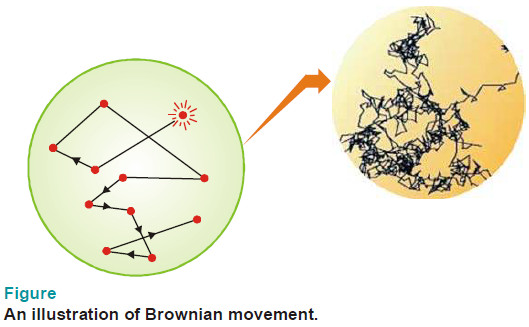
– The continuous rapid zig-zag movement executed by a colloidal particle in the dispersion medium is called Brownian movement or motion.
– This phenomenon is so named after Sir Robert Brown who discovered it in 1827.
– Suspension and true solutions do not exhibit Brownian movement.
Explanation of Brownian movement.
– The explanation of Brownian movement was advanced by Albert Einstein around 1955 by mathematical considerations based on the kinetic molecular theory.
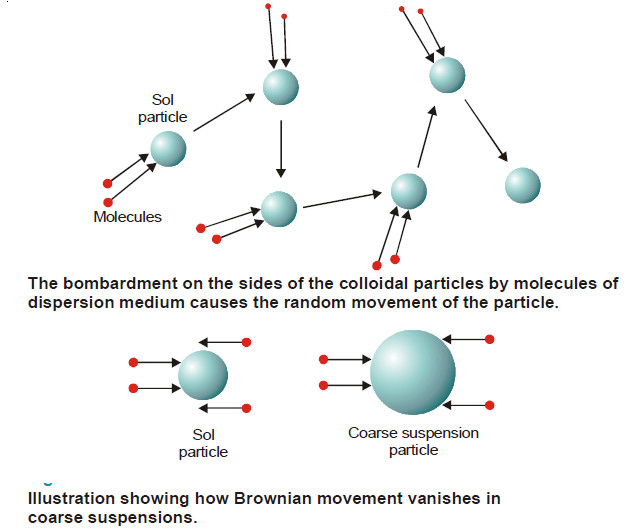
– According to him, at any instant a colloidal particle was being struck by several molecules of the dispersion medium.
– The movement of the particle was caused by unequal number of molecules of the medium striking it from opposite directions.
– When more molecules struck the particle on one side than on another, the direction of movement changed.
– The following figure illustrates how a colloidal particle is knocked about in a zig-zag path by molecules of the dispersion medium.
– In a suspension, the suspended particles being very large the probability of unequal bombardments diminishes.
– The force of the molecules hitting the particle on one side is cancelled by the force of collisions occurring on the other side. Hence they do not exhibit Brownian movement.
– The phenomenon of Brownian movement is an excellent proof of the existence of molecules and their ceaseless motion in liquids.
– It also explains how the action of gravity, which would ordinarily cause the settling of colloidal particles, is counteracted.
– The constant pushing of the particles by the molecules of the dispersion medium has a stirring effect which does not permit the particles to settle.