Nuclear Magnetic Resonance Imaging – NMR imaging
– In this topic, we will discuss Nuclear Magnetic Resonance Imaging.
Nuclear magnetic resonance imaging
– When chemists use NMR spectroscopy, they take great pains to get the most uniform magnetic field possible (often homogeneous to within one part per billion).
– They place small tubes of homogeneous solutions in the magnetic field and spin the tubes to average out any remaining variations in the magnetic field.
– Their goal is to have the sample behave as if it were all at a single point in the magnetic field, with every molecule subjected to exactly the same external magnetic field.
– Nuclear magnetic resonance imaging uses the same physical effect, but its goals are almost the opposite of chemical NMR.
– In NMR imaging, a heterogeneous sample (commonly a living human body) is placed in the magnetic field of a large-bore superconducting magnet.
– The magnetic field is purposely nonuniform, with a gradient that allows just the protons in one plane of the sample to be in resonance at any one time.
– By using a combination of field gradients and sophisticated Fourier transform techniques, the instrument can look selectively at one point within the sample, or a line within the sample, or a plane within the sample.
– The computer generates an image of a two-dimensional slice through the sample.
– A succession of slices can be accumulated in the computer to give a three-dimensional plot of the proton resonances within the bulk of the sample.
– Medical NMR imaging is commonly called magnetic resonance imaging (MRI) to avoid the common fear of the word nuclear and the misconception that “nuclear” means “radioactive.”
– There is nothing radioactive about an NMR spectrometer.
– In fact, MRI is the least invasive, least hazardous method available for imaging the interior of the body.
– The only common side effect is claustrophobia from being confined within the ring of the wide-bore magnet.
– The MRI image can easily distinguish watery tissues, fatty tissues, bone, air spaces, blood, etc. by their differences in composition and movement.
– By using proton relaxation times, the technique becomes even more useful.
– In a strong magnetic field, slightly more proton spins are aligned with the field (the lower-energy state) than against it.
– A radio-frequency pulse of just the right duration inverts some spins, increasing the number of spins oriented against the magnetic field.
– The spins gradually relax to their normal state over a period of a few seconds.
– By following the free-induction decay, the spectrometer measures how quickly spin relaxation occurs in each pixel of the sample.
– Differing relaxation times are coded by color or intensity in the image, giving valuable information about the tissues involved.
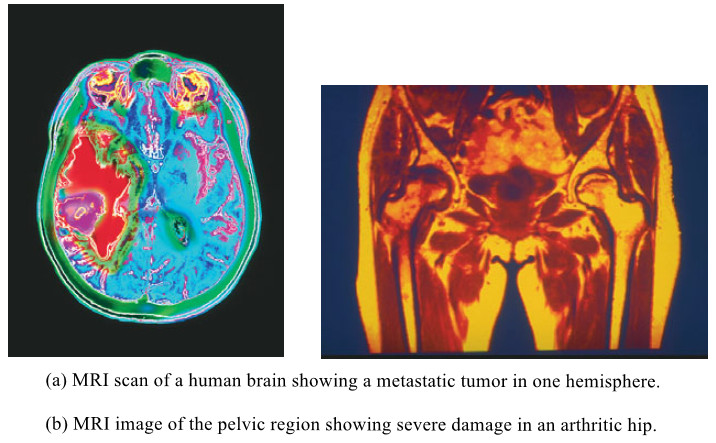
– For example, cancerous tissues tend to have longer relaxation times than the corresponding normal tissues, so tumors are readily apparent in the NMR image.
– The following Figure shows two actual MRI images:
- The first image is a slice through a patient’s head showing a brain tumor.
- The second image is a slice through another patient’s pelvic region showing an arthritic hip.
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.