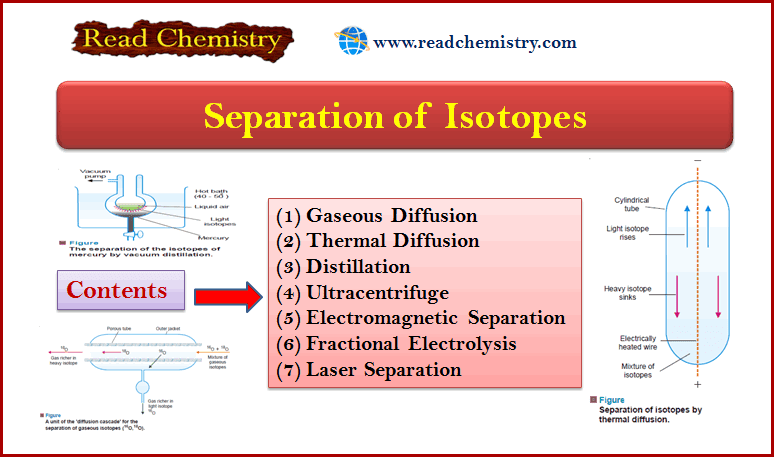
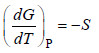Second Law of Thermodynamics: MCQ Quiz with answers
Second Law of Thermodynamics: MCQ Quiz with answers
– In this subject, you will find 49 questions and answers MCQ on The Second Law of Thermodynamics.
1. A process which proceeds of its own accord, without any outside assistance, is called ____ .
(a) non-spontaneous process
(b) spontaneous process
(c) reversible process
(d) irreversible process
Answer. (b)
2. The tendency of a process to occur naturally is called ____ .
(a) momentum of the reaction
(b) spontaneity of the reaction
(c) equilibrium of the reaction
(d) equilibrium of the reaction
Answer. (b)
3. Which of the following is true about the criteria of spontaneity?
(a) a spontaneous change is unidirectional
(b) a spontaneous change to occur, time is no factor
(c) once a system is in equilibrium, a spontaneous change is inevitable
(d) all of the above
Answer. (d)
4. A spontaneous change is accompanied by _____ of internal energy or enthalpy.
(a) increase
(b) decrease
(c) neither increase nor decrease
(d) none of these
Answer. (b)
5. Mixing of two or more gases is a ____ .
(a) spontaneous process
(b) non-spontaneous process
(c) reversible process
(d) none of these
Answer. (a)
6. Entropy is a measure of ___ of the molecules of the system.
(a) concentration
(b) velocity
(c) zig-zag motion
(d) randomness or disorder
Answer. (d)
7. The second law of thermodynamics states that ____ .
(a) whenever a spontaneous process occurs, it is accompanied by an increase in the total energy of the universe.
(b) the entropy of the system is constantly increasing
(c) neither of the above
(d) both (a) and (b)
Answer. (d)
8. The entropy of a pure crystal is zero at absolute zero. This is statement of ____ .
(a) first law of thermodynamics
(b) second law of thermodynamics
(c) third law of thermodynamics
(d) none of these
Answer. (c)
9. The entropy is measured in ____ .
(a) cal K–1 mol–1
(b) JK–1 mol–1
(c) entropy unit
(d) all of these
Answer. (d)
10. The standard entropy, S°, of a substance is ____ .
(a) its entropy at 0°C and 1 atm pressure
(b) its entropy at 0 K and 1 atm pressure
(c) its entropy at 25°C and 1 atm pressure
(d) its entropy at 25 K and 1 atm pressure
Answer. (c)
11. The change in entropy of a reaction is given by ____ .
(a) ΔS = ΣSReactants + ΣSProducts
(b) ΔS = ΣSProducts – ΣSReactants
(c) ΔS = ΣSReactants – ΣSProducts
(d) none of these
Answer. (b)
12. Which of the following is true for a cyclic process?
(a) ΔE = 0
(b) ΔE = q – w
(c) q = w
(d) all of these
Answer. (d)
13. A machine that can do work by using heat which flows out spontaneously from a high temperature source to a low-temperature sink is called ____ .
(a) Carnot machine
(b) cyclic machine
(c) heat machine
(d) heat engine
Answer. (d)
14. The efficiency of a heat engine is the ratio of ____ .
(a) work obtained in a cyclic process (w) to the heat taken from the high temperature reservoir (q).
(b) heat taken from the high temperature reservoir (q) to the work obtained in a cyclic process
(c) work obtained in a cyclic process (w) to the heat taken from the low temperature sink (q)
(d) none of the above
Answer. (a)
15. The cycle of processes which occurs under reversible conditions is referred to as ____ .
(a) cyclic process
(b) closed process
(c) Carnot cycle
(d) reversible cycle
Answer. (c)
16. The efficiency of a heat engine is given by ____ .
Answer. (a)
17. The second law of thermodynamics may be stated as ____ .
(a) it is impossible to take heat from a hotter reservoir and convert it completely into work by a cyclic process without transferring a part of heat to a cooler reservoir.
(b) it is impossible to transfer heat from a body at a lower temperature to one at higher temperature
(c) the efficiency of heat engine is always less than 1
(d) all of the above
Answer. (d)
18. The efficiency of a heat operating between 400 K and 300 K is ____ .
(a) 1.0
(b) 0.75
(c) 0.50
(d) 0.25
Answer. (d)
19. The efficiency of heat engine operating between 1000 K and 300 K is _______ the engine operating between 1000 K and 500 K.
(a) greater than
(b) lesser than
(c) is equal to
(d) none of these
Answer. (a)
20. The entropy of the system increases in the order ____ .
(a) gas < liquid < solid
(b) solid < liquid < gas
(c) gas < solid < liquid
(d) none of these
Answer. (b)
21. The efficiency of an irreversible Carnot cycle is always _______ that of a reversible one operating between the same two temperatures
(a) less than
(b) greater than
(c) equal to
(d) none of these
Answer. (a)
22. The free energy function (G) is defined as ____ .
(a) G = H + T S
(b) G = H – T S
(c) G = T S – H
(d) none of these
Answer. (b)
23. The change in free energy is a measure of ____ .
(a) net work done
(b) net change is entropy
(c) net change in enthalpy
(d) net change in internal energy
Answer. (a)
24. The work function (A) is defined as ____ .
(a) A = E – T S
(b) A = E + T S
(c) A = T S – E
(d) none of these
Answer. (a)
25. The change in free energy of a system is given by ____ .
(a) ΔG = ΔA + P ΔV
(b) ΔG = ΔH – T ΔS
(c) ΔG = ΔE + P ΔV – T ΔS
(d) all of these
Answer. (d)
26. Which out of the following is not a state function?
(a) free energy
(b) work function
(c) entropy
(d) work done
Answer. (d)
27. The variation of free energy with temperatures at constant pressure is given by the relation:
(a) dGP = – SdTP
(b)
(c) neither of these
(d) both (a) and (b)
Answer. (d)
28. The variation of free energy with pressure at constant temperature is given by ____ .
(a) (dG)T = V d PT
(b) dGP = – SdTP
(c)
(d) none of these
Answer. (a)
29. The change in free energy in an isothermal process for n moles of the gas is given by ____ .
(a) ΔG = 2.303 × n RT log P2/P1
(b) ΔG = 2.303 × RT log V1/V2
(c) ΔG = 2.303 × RT log P2/V1
(d) none of these
Answer. (a)
30. The Gibb’s Helmholtz equation is applicable to ____ .
(a) all processes, chemical or physical.
(b) all process, chemical or physical but in a closed system
(c) all chemical processes in a closed system
(d) all physical processes in a closed system
Answer. (b)
31. For a spontaneous process ____ .
(a) ΔG > 0
b) ΔG < 0
(c) ΔG = 0
(d) none of these
Answer. (b)
32. A process is in the equilibrium state when ____ .
(a) ΔG > 0
(b) ΔG < 0
(c) ΔG = 0
(d) none of these
Answer. (c)
33. Which of the following equation is used to calculate the heats of reaction when ΔG at two temperature are given?
(a) Gibbs Helmholtz equation
(b) Clapeyron equation
(c) Kirchoff’s equation
(d) none of these
Answer. (a)
34. The equation 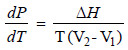
(a) Gibb’s Helmholtz equation
(b) Kirchoff’s equation
(c) Clapeyron equation
(d) Clausius Clapeyron equation
Answer. (c)
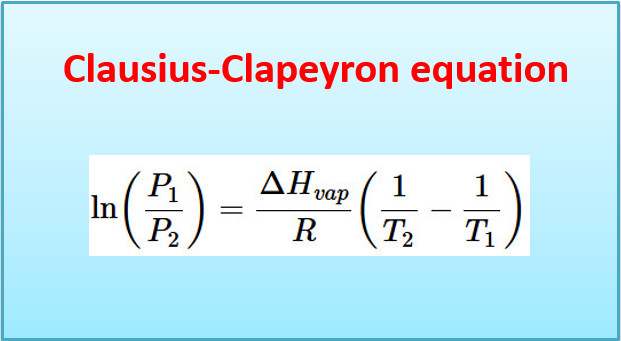
35. The Clausius Clapeyron equation helps to calculate ____ .
(a) latent heat of vaporization
(b) boiling point or freezing point
(c) vapour pressure at one temperature, if at another temperature is given
(d) all of the above
Answer. (d)
36. The decrease in the work function A in any process at constant temperature gives the _______ that can be obtained from the system during any change.
(a) minimum work
(b) maximum work
(c) useful work
(d) net work
Answer. (b)
37. The equation for van’t Hoff isotherm is ____ .
(a) –ΔG = 2.303 RT log KP
(b) ΔG = 2.303 RT log KP
(c) –ΔG = 2.303 RT2 log KP
(d) ΔG = 2.303 RT2 log KP
Answer. (a)
38. The equation is known as ____ .
(a) van’t Hoff equation
(b) van’t Hoff isochore
(c) Gibbs equation
(d) Gibbs Duhem equation
Answer. (b)
39. Each substance in a given state has a tendency to escape from that state and this escaping tendency is called ____ .
(a) spontaneity
(b) Gibbs free energy
(c) fugacity
(d) activity
Answer. (c)
40. When water is cooled to ice, its entropy ____ .
(a) increases
(b) decreases
(c) remains the same
(d) becomes zero
Answer. (b)
41. Which of the following sets of conditions makes a process spontaneous at all temperatures?
(a) ΔH = 0; ΔS > 0
(b) ΔH = 0; ΔS < 0
(c) ΔH > 0; ΔS > 0
(d) ΔH < 0; ΔS < 0
Answer. (c)
42. The increase in entropy is maximum in ____ .
(a) CaCO3 (s) → CaO (s) + CO2 (g)
(b) CO (g) + ½O2 (g) → CO2 (g)
(c) N2 (g) + 3H2 (g) → 2NH3 (g)
(d) H2 (g) + I2 (g) → 2HI (g)
Answer. (a)
43. A spontaneous reaction proceeds with a decrease in ____ .
(a) entropy
(b) enthalpy
(c) free energy
(d) internal energy
Answer. (c)
44. In a process ΔH = 100 kJ and ΔS = 100 JK–1 at 400 K. The value of ΔG will be ____ .
(a) zero
(b) 100 kJ
(c) 50 kJ
(d) 60 kJ
Answer. (d)
45. A chemical reaction proceeds with decrease in both the enthalpy and entropy. This reaction will be spontaneous if ____ .
(a) ΔH = T ΔS
(b) ΔH < T ΔS
(c) ΔH > T ΔS
(d) none of these
Answer. (b)
46. Which is the correct unit for entropy?
(a) kJ mol
(b) JK–1 mol
(c) JK–1 mol–1
(d) kJ mol
Answer. (c)
47. The efficiency of heat engine is maximum when ____ .
(a) temperatures of source and sink are maximum
(b) temperatures of source and sink are minimum
(c) temperature of source is minimum and that of sink is maximum
(d) temperature of source is maximum and that of sink is minimum
Answer. (d)
48. A reaction proceeds with increase in both the enthalpy and entropy. It will be spontaneous if ____ .
(a) ΔH = T ΔS
(b) ΔH > T ΔS
(c) ΔH < T ΔS
(d) none of these
Answer. (c)
49. A spontaneous reaction is not possible if ____ .
(a) ΔH and T ΔS are both negative
(b) ΔH and T ΔS are both positive
(c) ΔH is +ve and T ΔS is –ve
(d) ΔH is –ve and T Δ S is +ve
Answer. (c).