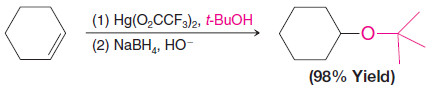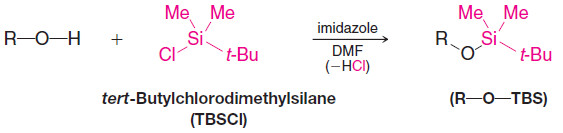Synthesis of Ethers
Synthesis of Ethers
– In this topic, we will discuss 6 methods for Synthesis of Ethers as follows:
1. Ethers by Intermolecular Dehydration of Alcohols
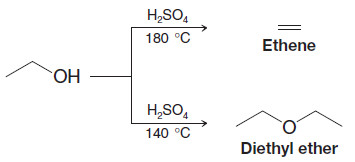
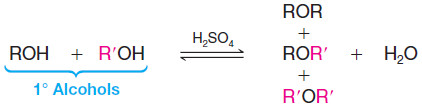
– Two alcohol molecules can form an ether by loss of water through an acid-catalyzed substitution reaction.
– This reaction competes with the formation of alkenes by acid-catalyzed alcohol dehydration.
– Intermolecular dehydration of alcohols usually takes place at lower temperature than dehydration to an alkene, and dehydration to the ether can be aided by distilling the ether as it is formed.
– For example, diethyl ether is made commercially by dehydration of ethanol.
– Diethyl ether is the predominant product at 140 oC; ethene is the predominant product at 180oC.
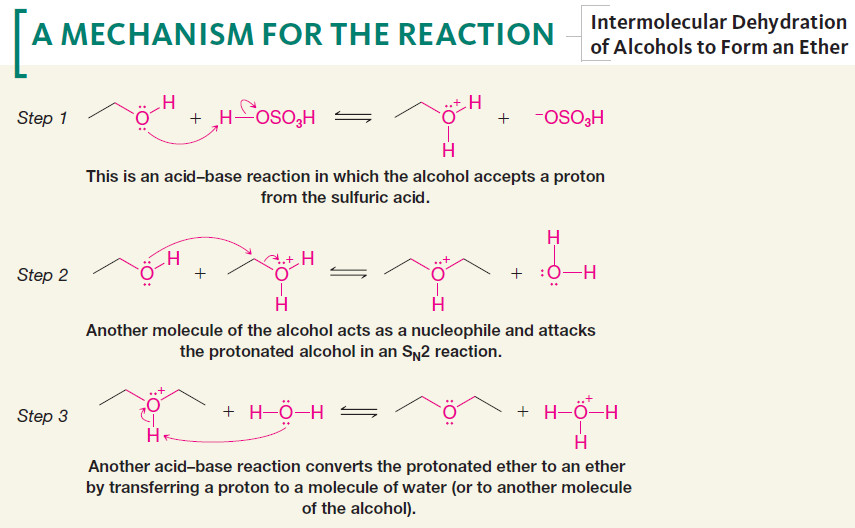
– The formation of the ether occurs by an SN2 mechanism with one molecule of the alcohol acting as the nucleophile and another protonated molecule of the alcohol acting as the substrate.
Mechanism of Intermolecular Dehydration of Alcohols to Form an Ether
Complications of Intermolecular Dehydration
– The method of synthesizing ethers by intermolecular dehydration has some important limitations.
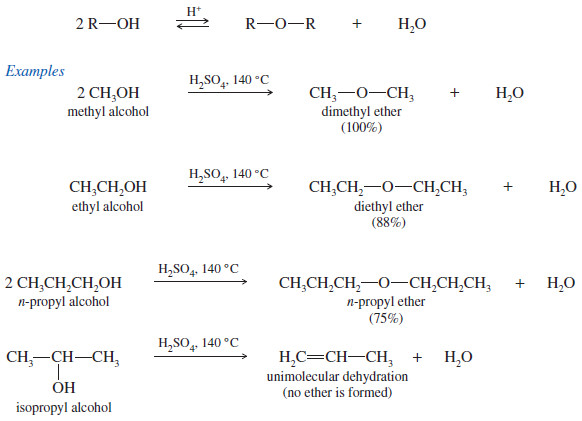
(1) Attempts to synthesize ethers by intermolecular dehydration of secondary alcohols are usually unsuccessful because alkenes form too easily.
(2) Attempts to make ethers with tertiary alkyl groups lead predominantly to alkenes
(3) Intermolecular dehydration is not useful for the preparation of unsymmetrical ethers from primary alcohols because the reaction leads to a mixture of products.
Examples of Intermolecular Dehydration of Alcohols to Form an Ether
Note: This nethod also named Industrial Synthesis: Bimolecular Condensation of Alcohols.
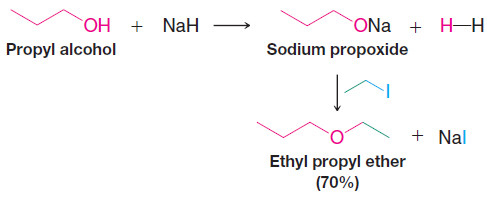
2. The Williamson Ether Synthesis
– An important route to unsymmetrical ethers is a nucleophilic substitution reaction known as the Williamson ether synthesis.
– The Williamson ether synthesis consists of an SN2 reaction of a sodium alkoxide with an alkyl halide, alkyl sulfonate, or alkyl sulfate.
– The following reaction is a specific example of the Williamson ether synthesis. The sodium alkoxide can be prepared by allowing an alcohol to react with NaH:
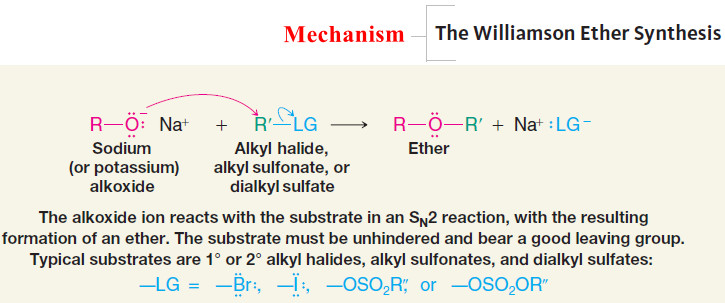
Mechanism of The Williamson Ether Synthesis
– The usual limitations of SN2 reactions apply here:
– Best results are obtained when the alkyl halide, sulfonate, or sulfate is primary (or methyl). If the substrate is tertiary, elimination is the exclusive result.
– Substitution is also favored over elimination at lower temperatures.
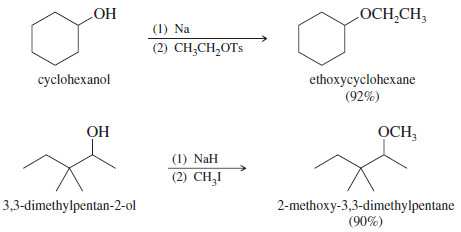
Examples
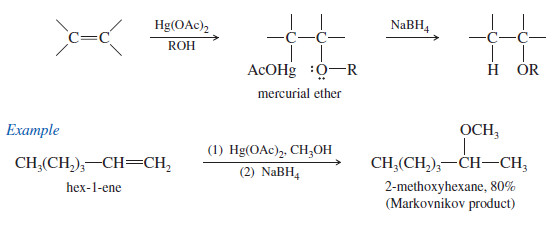
3. Synthesis of Ethers by Alkoxymercuration–Demercuration
– Alkoxymercuration–demercuration is another method for synthesizing ethers.
– The reaction of an alkene with an alcohol in the presence of a mercury salt such as mercuric acetate or trifluoroacetate leads to an alkoxymercury intermediate, which on reaction with sodium borohydride yields an ether.
– When the alcohol reactant is also the solvent, the method is called solvomercuration– demercuration.
– This method directly parallels hydration by oxymercuration–demercuration
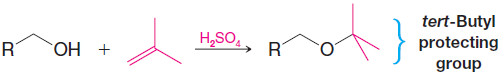
4. tert-Butyl Ethers by Alkylation of Alcohols: Protecting Groups
– Primary alcohols can be converted to tert-butyl ethers by dissolving them in a strong acid such as sulfuric acid and then adding isobutylene to the mixture. (This procedure minimizes dimerization and polymerization of the isobutylene.)
– A tert-butyl ether can be used to “protect” the hydroxyl group of a primary alcohol while another reaction is carried out on some other part of the molecule.
– A tert-butyl protecting group can be removed easily by treating the ether with dilute aqueous acid.
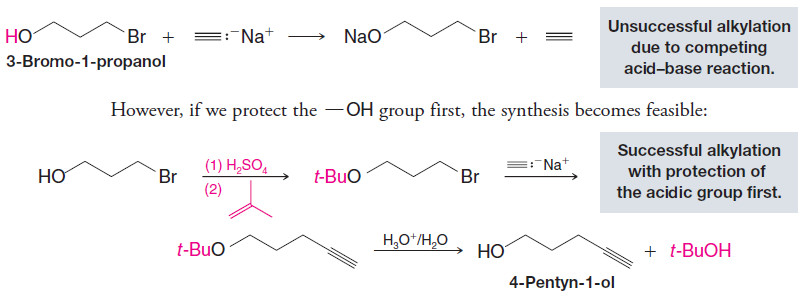
– Suppose, for example, we wanted to prepare 4-pentyn-1-ol from 3-bromo-1-propanol and sodium acetylide.
– If we allow them to react directly, the strongly basic sodium acetylide will react first with the hydroxyl group, making the alkylation unsuccessful:
5. Silyl Ether Protecting Groups
– A hydroxyl group can be protected from acid–base reactions by converting it to a silyl ether group.
– One of the most common silyl ether protecting groups is the tert-butyldimethylsilyl ether group [tert-butyl (Me)2Si-O-R, or TBS-O-R], although triethylsilyl, triisopropylsilyl, tert butyldiphenylsilyl, and others can be used.
– The tert-butyldimethylsilyl ether is stable over a pH range of roughly 4–12.
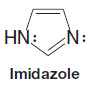
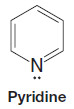
– A TBS group can be added by allowing the alcohol to react with tert butyldimethylsilyl chloride in the presence of an aromatic amine (a base) such as imidazole or pyridine:
– The TBS group can be removed by treatment with fluoride ion (tetrabutylammonium fluoride or aqueous HF is frequently used).
– These conditions tend not to affect other functional groups, which is why TBS ethers are such good protecting groups.
– Converting an alcohol to a silyl ether also makes it much more volatile.
– This increased volatility makes the alcohol (as a silyl ether) much more amenable to analysis by gas chromatography. Trimethylsilyl ethers are often used for this purpose.
– The trimethylsilyl ether group is too labile to use as a protecting group in most reactions, however.
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.