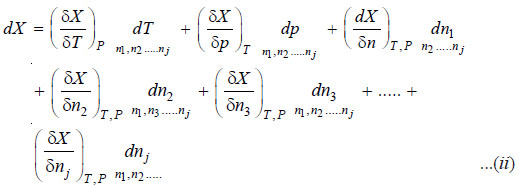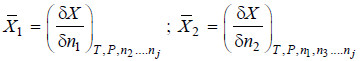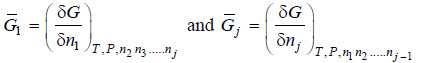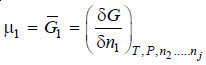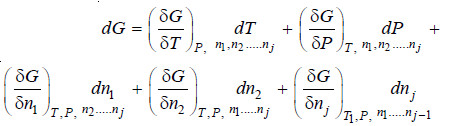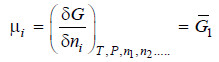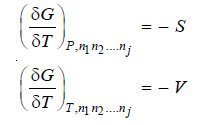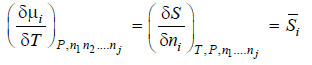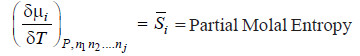Chemical Potential
– In this topic, we will discuss The Chemical Potential and Variation of Chemical Potential with Temperature and Pressure.
Partial Molar Properties
– We have so far studied the thermodynamic systems in which there was a change in thermodynamic properties with the variation of one or more state variables.
– In such systems there was no transfer of mass taking place (closed systems).
– For studying the systems containing two or more phases or components G.N. Lewis introduced the concept of partial molar properties as in these cases both mass and composition vary (open systems).
– Consider any extensive thermodynamic property X of such a system, the value of which is determined by the temperature, pressure and the amounts of various constituents present.
– Let the system consist of J constituents and let n1, n2, n3 …. nj be the number of moles of the various constituents present.
– Evidently X must be a function of P, T and the number of moles of various constituents present, i.e.
…(1)
– If there is a small change in the temperature and pressure of the system as well as the amounts of
its constituents, the change in the property X is given by:
– The quantity 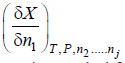
– It is represented by writing a bar over its symbol for the particular property i.e. so that
– The equation (ii) may be written as:
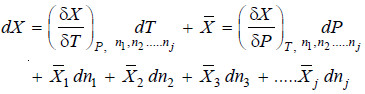
– If the temperature and the pressure of the system are kept constant dT and dP are zero so that
..(iii)
and this on integration for a system of definite composition represented by the number of moles n1, n2 ….. nj gives
…(iv)
– i.e., the partial molal property of any constituent may be regarded as the contribution of 1 mole of that constituent to the total value of the property of the system under specified conditions.
Partial Molar Free Energy : Chemical Potential
– If the extensive property under study is free energy (G), will represent the partial molar free energy so that
– This quantity is, for most purposes, identical with the function known as chemical potential represented by the symbol μ. Accordingly we have
Thus, it is the partial derivative of the free energy with ni when all other variables are kept constant.
Physical Significance of Chemical Potential
– By definition the chemical potential of a given substance is the change in free energy of the system produced on addition of one mole of the substance at constant temperature and pressure to a large bulk of the mixture so that its composition does not undergo any change.
– It is an intensive property and it may be regarded as the force which drives the chemical system to equilibrium.
– At equilibrium the chemical potential of the substance in the system must have the same value through the system.
– In other words, the matter flows spontaneously from a region of high chemical potential to low chemical potential.
– The chemical potential may also be regarded as the escaping tendency of that system.
– Greater the chemical potential of a system greater will be its escaping tendency.
Gibbs Duhem Equation
– It has already been discussed that free energy G is an intensive thermodynamic property.
– It can be determined by fixing the variables T, P and number of moles of various constituents (composition of the mixture under study).
– Mathematically, we can write.
…(i)
where n1, n2 ……. nj are the number of moles of various constituents.
– Differentiating equation (i), we get
– We know the chemical potential is given by
– Substituting in equation (ii) we get
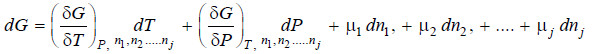
– For a closed system there is no change in the composition and equation (iii) reduces to
– comparing equation (iv) and (v)
– Putting these values in equation (iii) we get
..(vi)
– At constant temperature and pressure equation (vi) reduces to
…(vii)
– Integrating equation (vii) we get the following for a system of definite composition
… (viii)
– Differentiating equation (viii) we get
… (ix)
– Comparing equation (vii) and (ix) we get
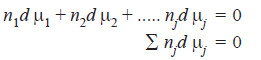
– Equation (x) is called Gibbs Duhem equation.
– It is applicable to a system at constant temperature and pressure.
Variation of Chemical Potential with Temperature and Pressure
(a) With Temperature
– We know that chemical potential of a constituent i in a system is given by
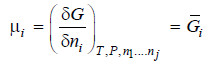
– Differentiating equation (i) w.r.t. T at constant pressure P, we get
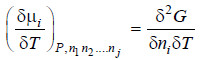
– We also know that
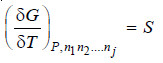
– Differentiating equation (iii) w.r.t. nj at constant temperature and pressure.
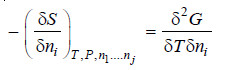
– Comparing equation (ii) and (iv), we have
[By definition of Partial Molal Property]
– Thus
– This equation gives the variation of chemical potential of any constituent i with temperature.
(b) With Pressure
– By definition, chemical potential is given by
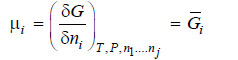
– Differentiating equation (i) w.r.t. pressure at constant temperature
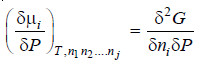
– But we know that

– Differentiating equation (iii) w.r.t. ni at constant T, P and nj
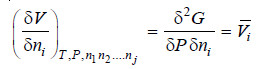
(By definition of Partial Molal Property)
– Comparing equations (ii) and (iv)
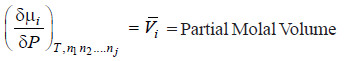
– Equation (v) gives the variation of Chemical potential of any constituent i with pressure.
– From this equation we can define the partial molar volume of a constituent i as the rate of change of chemical potential of a constituent i with pressure at constant temperature.
References
- Atkins’ Physical Chemistry / Peter Atkin, Julio de Paula, James Keeler / 12th edition, 2022 / Oxford University Press, UK.
- Physical Chemistry/ Robert G. Mortimer/ 3rd Edition / 2008/ Elsevier Inc, USA.
- Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition/ S. Chand Publishing co / india.
- Physical chemistry for the chemical sciences / Raymond Chang, John W. Thoman, Jr./1st edition, 2014/ University Science Books, USA