Enthalpy: Heats of Reaction and Chemical Change
In this subject, we will discuss the Enthalpy: Heats of Reaction and Chemical Change.
Enthalpy: Heats of Reaction and Chemical Change
– Most physical and chemical changes occur at virtually constant atmospheric pressure—a reaction in an open flask, the freezing of a lake, a drug response in an organism.
– In this subject, we define a thermodynamic variable that makes it much easier to measure energy changes at constant pressure.
The Meaning of Enthalpy
– To determine ΔE, we must measure both heat and work.
– The two most important types of chemical work are electrical work, the work done by moving charged particles, and PV work, the work done by an expanding gas.
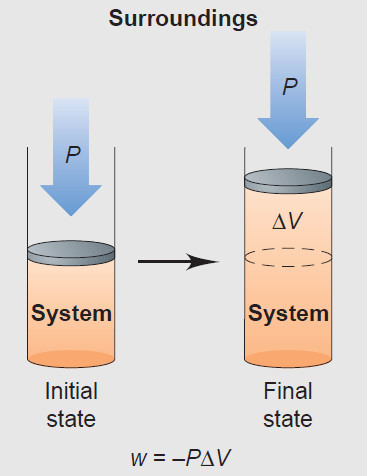
– We find the quantity of PV work done by multiplying the external pressure (P) by the change in volume of the gas (ΔV, or Vfinal – Vinitial).
– In an open flask (or a cylinder with a weightless, frictionless piston), a gas does work by pushing back the atmosphere.
– Work done on the surroundings is a negative quantity because ΔV is positive; work done on the system is a positive quantity because ΔV is negative:
w = – PΔV
– For reactions at constant pressure, a thermodynamic variable called enthalpy (H) eliminates the need to consider PV work separately.
– The enthalpy of a system is defined as the internal energy plus the product of the pressure and volume:
H = E + PV (1)
– The change in enthalpy (ΔH) is the change in internal energy plus the product of the constant pressure and the change in volume:
ΔH + ΔE = PΔV (2)
– Combining Equation (ΔE = q + w) and Equation (1) leads to a key point about ΔH:
ΔE = q + w = q + (- PΔV) = q – PΔV
– At constant pressure, we denote q as qP and solve for it:
qP = ΔE + PΔV
– Notice the right side of this equation is identical to the right side of Equation (2):
qP = ΔE + PΔV = ΔH
– Thus, the change in enthalpy equals the heat gained or lost at constant pressure.
– Since most changes occur at constant pressure, ΔH is more relevant than ΔE and easier to find: to find ΔH, measure qP.
Exothermic and Endothermic Processes
– Because E, P, and V are state functions, H is also a state function, which means that ΔH depends only on the difference between Hfinal and Hinitial.
– The enthalpy change of a reaction, also called the heat of reaction, ΔHrxn, always refers to Hfinal minus Hinitial:
ΔH = Hfinal – Hinitial = Hproducts – Hreactants
– Therefore, because Hproducts can be either more or less than Hreactants, the sign of ΔH indicates whether heat is absorbed or released in the change.
– We determine the sign of ΔH by imagining the heat as a “reactant” or “product.”
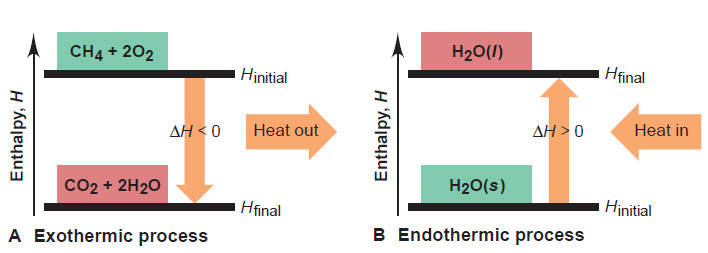
– When methane burns in air, for example, we know that heat is produced, so we show it as a product (on the right):
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g) + heat
– Because heat is released to the surroundings, the products (1 mol of CO2 and 2 mol of H2O) must have less enthalpy than the reactants (1 mol of CH4 and 2 mol of O2).
– Therefore, ΔH (Hfinal – Hinitial) is negative, as the enthalpy diagram in Figure (A) shows.
In Exothermic Process
– An exothermic (“heat out”) process releases heat and results in a decrease in the enthalpy of the system:
Exothermic:
Hfinal < Hinitial ΔH < 0 (ΔH is negative)
In Endothermic Process
– An endothermic (“heat in”) process absorbs heat and results in an increase in the enthalpy of the system.
– When ice melts, for instance, heat flows into the ice from the surroundings, so we show the heat as a reactant (on the left):
heat + H2O(s)→ H2O(l)
– Because heat is absorbed, the enthalpy of the liquid water is higher than that of the solid water, as Figure (B) shows. Therefore, ΔH (Hwater – Hice) is positive:
Endothermic:
Hfinal < Hinitial ΔH >0 (ΔH is positive)
– In general, the value of an enthalpy change refers to reactants and products at the same temperature.
Summary: Enthalpy- Heats of Reaction and Chemical Change
– The change in enthalpy, ΔH, is equal to the heat lost or gained during a chemical or physical change that occurs at constant pressure, qP.
– A change that releases heat is exothermic (ΔH < 0); a change that absorbs heat is endothermic (ΔH > 0).
References
- Principles of Inorganic Chemistry / Brian W. Pfennig / 1st ed, 2015 /John Wiley & Sons, Inc/ USA.
- Principles of general Chemistry / Martin S. Silberberg / 2nd ed, 2010 /The McGraw-Hill Companies, Inc./ USA.
- Inorganic Chemistry /Peter Atkins, Tina Overton, Jonathan Rourkel, Mark Weller, Fraser Armstrong, Mike Hagerman / 6th ed, 2014 /W. H. Freeman and Company/ New York, USA.
-