General Chemistry
General Chemistry introduces basic chemical concepts, including atomic structure, bonding, reactions, stoichiometry, states of matter, and periodic trends. It lays the groundwork for advanced study in all chemistry branches.
-
Fundamentals of Chemistry book by Romain Elsair – Free download
– In this subject, we will discuss the free download of Fundamentals of Chemistry book by Romain Elsair. Aim of…
Read More » -
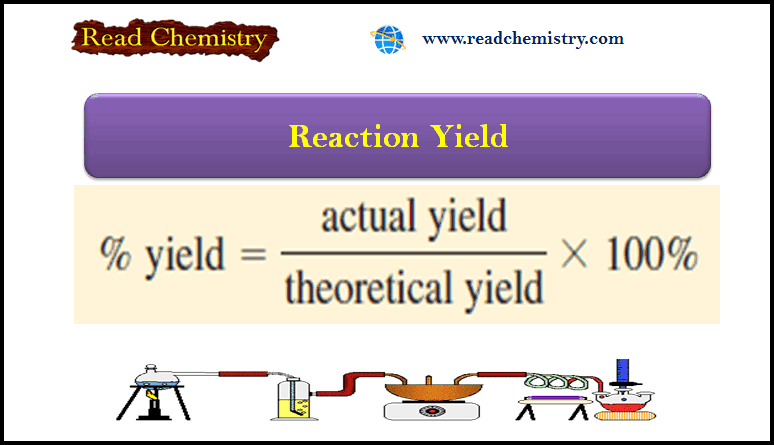
Reaction Yield – How to Calculate Reaction Yield?
Reaction Yield – The amount of limiting reagent present at the start of a reaction determines the theoretical yield of…
Read More » -
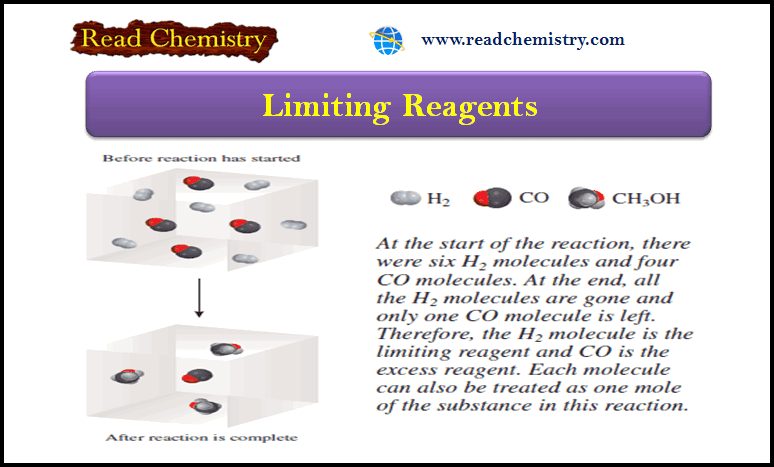
Limiting Reagent: Definition, Examples, Problems
– In this subject, we will discuss the Limiting Reagent (Definition, Examples, Problems) Limiting Reagent – When a chemist carries…
Read More » -
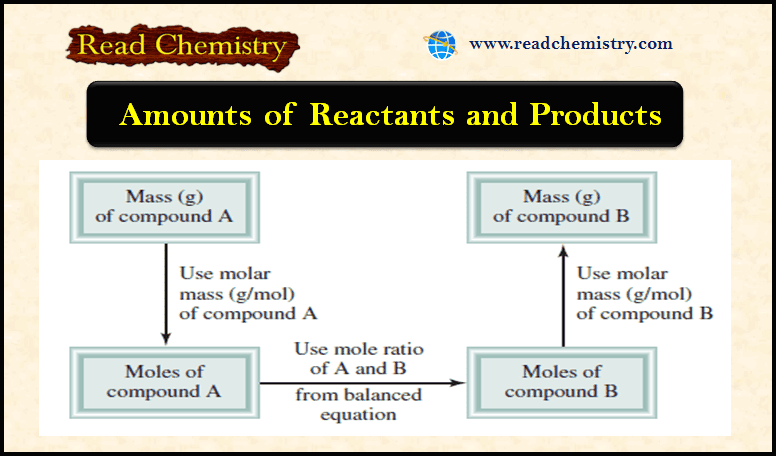
Amounts of Reactants and Products
– In this subject, we will discuss Amounts of Reactants and Products Amounts of Reactants and Products – A basic…
Read More » -
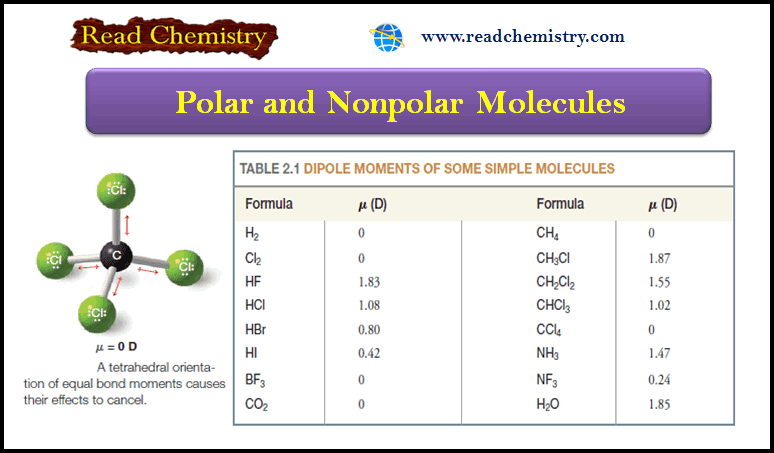
Polar and Nonpolar Molecules
– In this subject, we will discuss the Polar and Nonpolar Molecules. Dipole moment – The dipole moment is a…
Read More » -
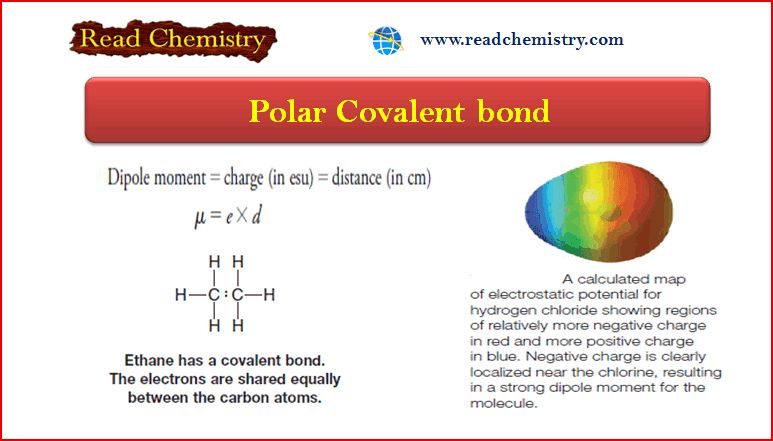
Polar Covalent Bond and Dipole moment
– In this subject, we will discuss the Polar Covalent Bond and Dipole moment. Polar Covalent Bonds – Covalent bonds…
Read More » -
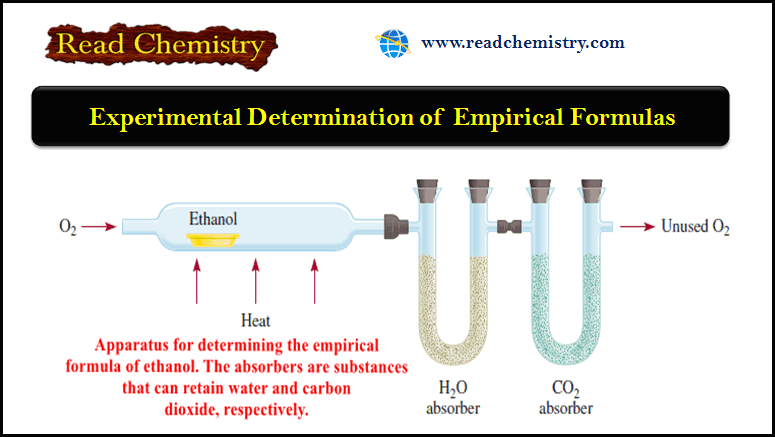
Experimental Determination of Empirical Formulas
Experimental Determination of Empirical Formulas ** The fact that we can determine the empirical formula of a compound if…
Read More » -
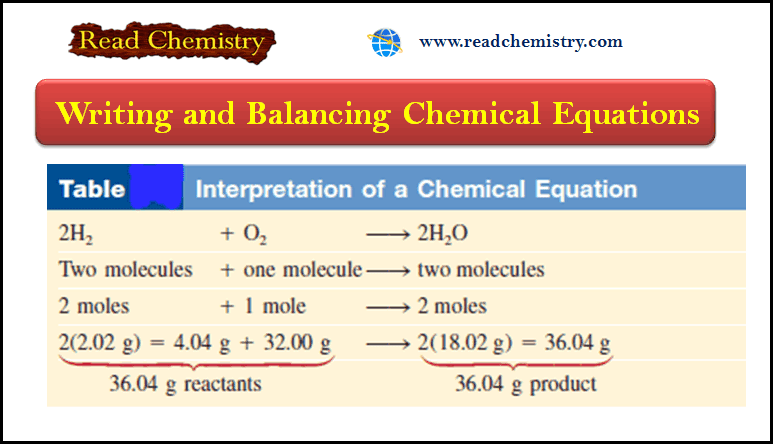
Chemical Equations – Writing and Balancing Chemical Equations
– In this subject, we will discuss Writing and Balancing Chemical Equations. Chemical Reactions and Chemical Equations – A chemical…
Read More » -
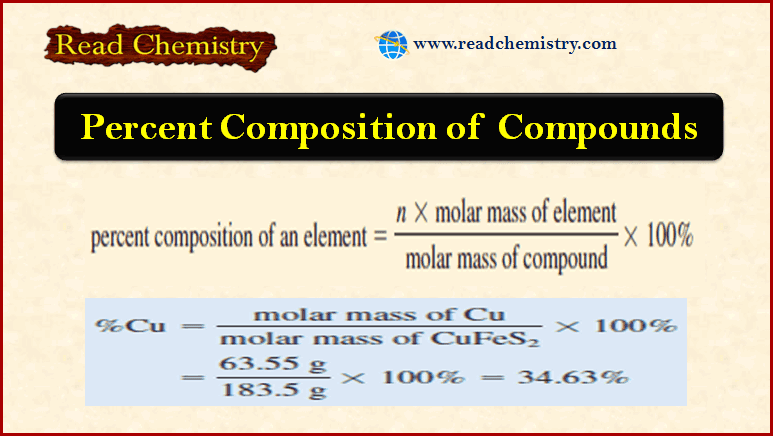
Percent Composition of Compounds
Percent Composition of Compounds **As we have seen, the formula of a compound tells us the numbers of atoms…
Read More » -
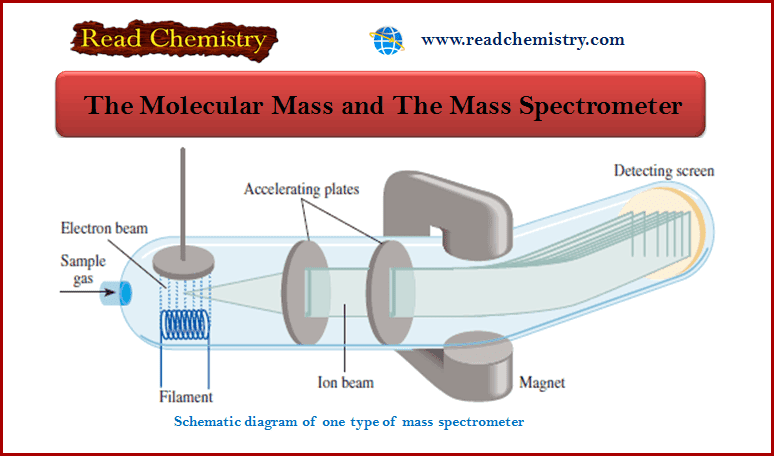
The Molecular Mass and The Mass Spectrometer
Molecular Mass ** If we know the atomic masses of the component atoms, we can calculate the mass of…
Read More » -
Avogadro’s Number and the Molar Mass of an Element
Avogadro’s Number (NA) ** Atomic mass units provide a relative scale for the masses of the elements. ** But because…
Read More » -
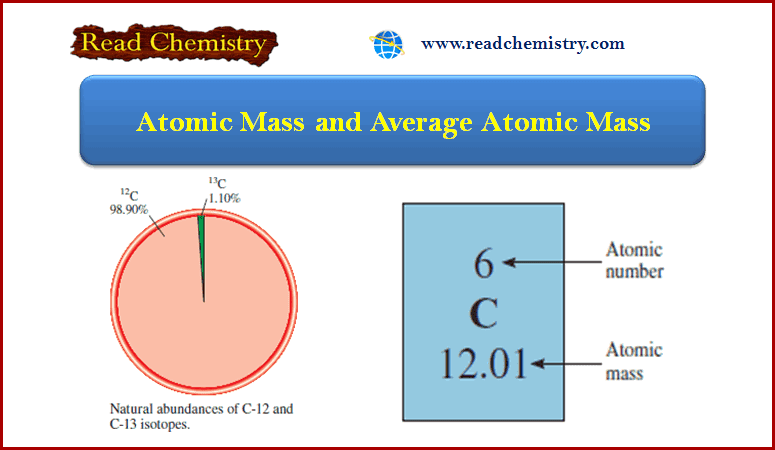
Atomic Mass and Average Atomic Mass: Definition, Calculation
– In this subject, we will discuss the Atomic Mass and Average Atomic Mass: Definition, Formula, and Calculation Atomic Mass…
Read More » -
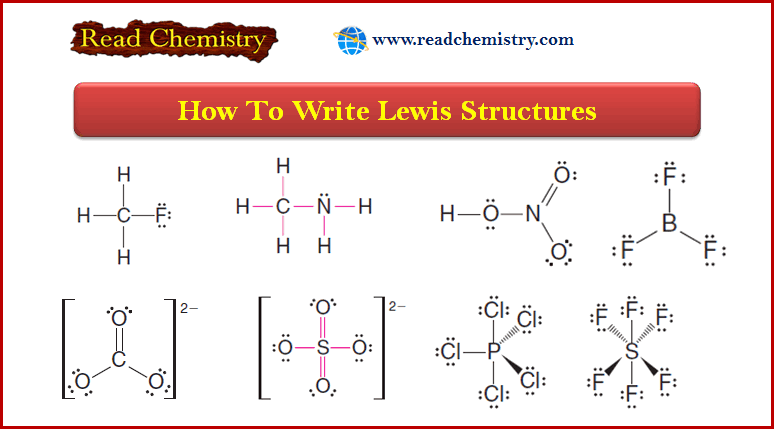
Lewis Structures: Definition, Structural Formula, Examples
– In this subject, we will discuss the Lewis Structures: Definition, Overview, Structural Formula, Examples Definition of Lewis structures –…
Read More » -
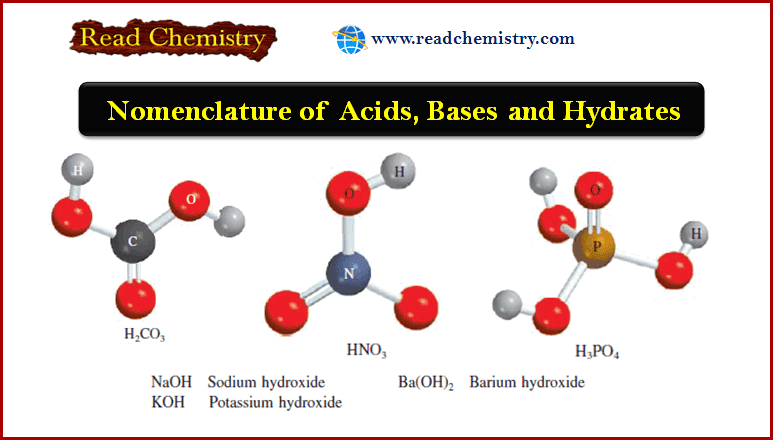
Nomenclature of Acids, Bases and Hydrates
– In this subject, we will discuss the Nomenclature of Acids, Bases and Hydrates Nomenclature of Acids – An acid…
Read More » -
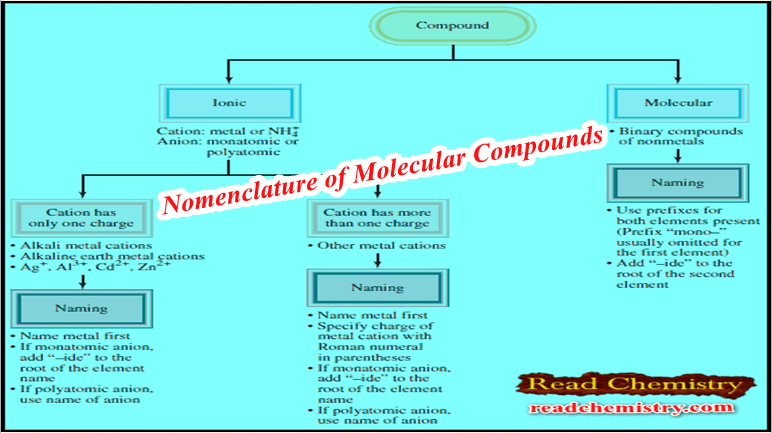
Nomenclature of Molecular Compounds
How to Name Molecular Compounds – Unlike ionic compounds, Molecular compounds contain discrete molecular units. – They are usually composed…
Read More » -
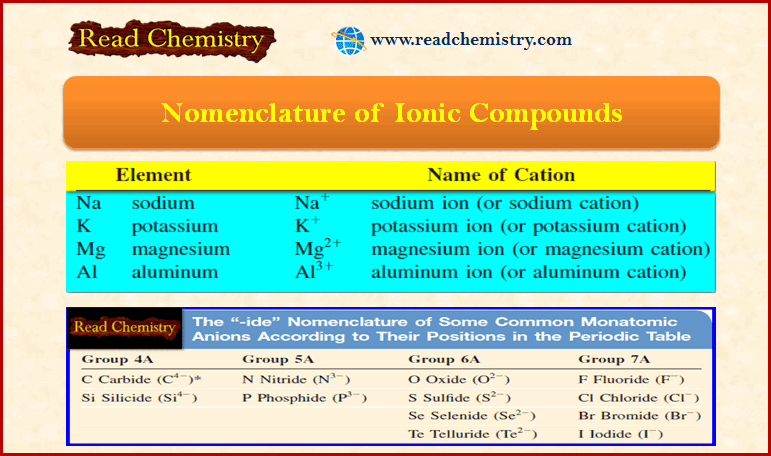
Nomenclature of Ionic Compounds
– In this subject, we will discuss the Nomenclature of Ionic Compounds. Naming Compounds – In addition to using formulas…
Read More » -
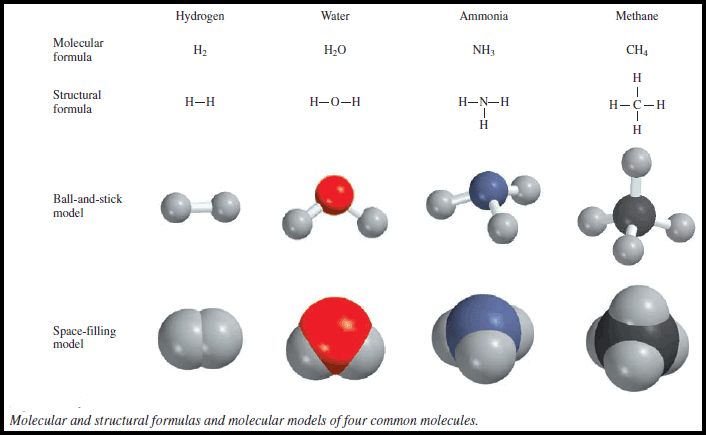
Molecular Formulas and Empirical Formulas: Definitions, Examples
– In this subject, we will discuss Molecular Formulas and Empirical Formulas: Definitions, Examples – Chemists use Chemical formulas to express…
Read More » -
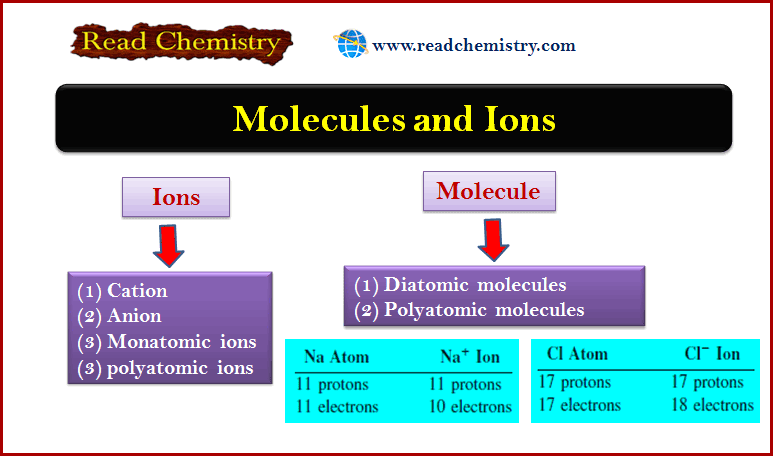
Atoms, Molecules, and Ions: Definition, Types, Examples
– In this subject, we will discuss the Atoms, Molecules, and Ions: Definition, Types, Examples Atoms – Of all the…
Read More » -
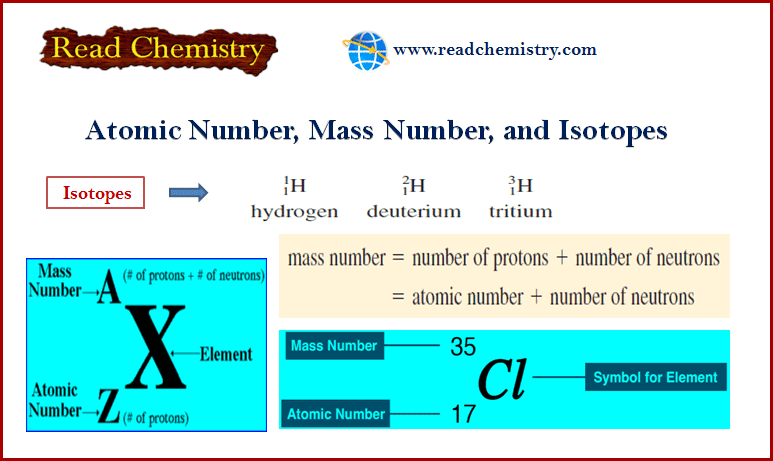
Atomic Number, Mass Number, and Isotopes
– In this subject, we will discuss the Atomic Number, Mass Number, and Isotopes: Definition, Formula, and Calculation Atomic number…
Read More » -
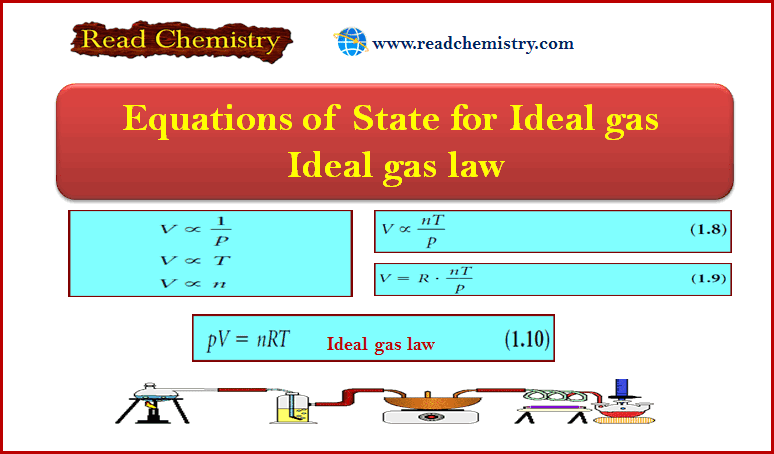
Equations of State for Ideal gas – Ideal gas law
Equations of State for Ideal gas ** Phenomenological thermodynamics is based on experiment, on measurements that you might make…
Read More »