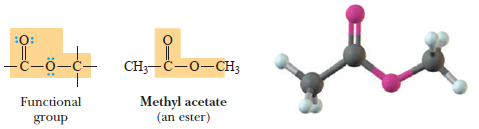Functional Groups in Organic Chemistry
– In this subject, we will discuss the Functional Groups in Organic Chemistry.
Functional Groups
– Carbon combines with other atoms (e.g., H, N, O, S, halogens) to form structural units called functional groups.
– Functional groups are important for three reasons.
– First, they are the units by which we divide organic compounds into classes.
– Second, they are sites of characteristic chemical reactions.
– A particular functional group, in all compounds that contain it, undergoes the same types of chemical reactions.
– In addition to the reason up, functional groups serve as a basis for naming organic compounds. therefore there are a lot of Functional groups.
– Hence, We introduce here several of the functional groups.
– We shall have more to say about the structure and properties of these functional groups in the following subjects on our website.
(1) Alcohols
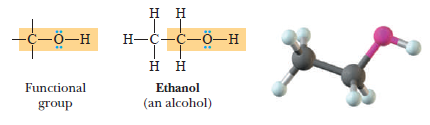
– The functional group of an alcohol is an -OH (hydroxyl) group bonded to a tetrahedral carbon atom (a carbon having single bonds to four other atoms).
– Here is the Lewis structure of ethanol.
– We can also represent this alcohol in a more abbreviated form called a condensed structural formula.
– In a condensed structural formula, CH3 indicates a carbon bonded to three hydrogens, CH2 indicates a carbon bonded to two hydrogens, and CH indicates a carbon bonded to one hydrogen.
– In a condensed structural formula, we can write an alcohol formula without Unshared pairs of electrons(Oxygen).
– Thus, the condensed structural formula for the alcohol with molecular formula C2H6O is CH3-CH2-OH.
– It is also common to write these formulas in an even more condensed manner, by omitting all single bonds: CH3CH2OH.
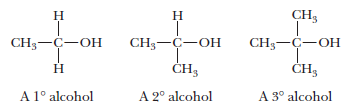
Primary alcohols (1°)
– A compound containing a functional group bonded to a carbon atom bonded to only one other carbon atom and two hydrogens.
Secondary alcohols (2°)
– A compound containing a functional group bonded to a carbon atom bonded to two other carbon atoms and one hydrogen atom.
Tertiary alcohols (3°)
– A compound containing a functional group bonded to a carbon atom bonded to three other carbon atoms.
(2) Amines
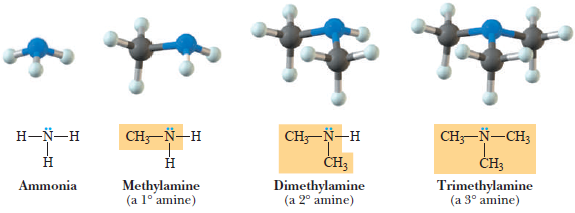
– The functional group of an amine is an amino group, a nitrogen atom bonded to one, two, or three carbon atom(s) by single bonds.
– In a primary (1°) amine, nitrogen is bonded to one carbon atom.
– In a secondary (2°) amine, it is bonded to two carbon atoms, and in a tertiary (3°) amine, it is bonded to three carbon atoms.
– Notice that this classification scheme is different from that used with alcohols and halides.
Amino group
– A compound containing a nitrogen atom bonded to one, two, or three carbon atom(s) by single bonds.
Primary (1o) amine
– An amine in which nitrogen is bonded to one carbon and two hydrogens.
Secondary (2o) amine:
– An amine in which nitrogen is bonded to two carbons and one hydrogen.
Tertiary (3o) amine:
– An amine in which nitrogen is bonded to three carbons.
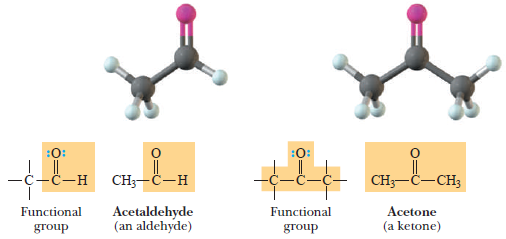
(3) Aldehydes and Ketones
– The functional group of both aldehydes and ketones is the C=O (carbonyl) group.
– Formaldehyde, CH2O, is the simplest aldehyde, the carbonyl carbon is bonded to two hydrogens.
– In all other aldehydes, it is bonded to one hydrogen and one carbon.
– a condensed structural formula, the aldehyde group may be written showing the carbon-oxygen double bond as -CH=O; alternatively, it may be written -CHO.
– In a ketone, the carbonyl carbon is bonded to two carbon atoms.
Carbonyl group
– It is C = O group.
Aldehyde
– A compound containing a -CHO group.
Ketone
– A compound containing a carbonyl group bonded to two carbons.
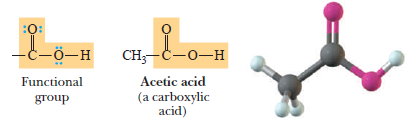
(4) Carboxylic Acids
– The functional group of a carboxylic acid is a -COOH (carboxyl: carbonyl+ hydroxyl) group.
– In a condensed structural formula, we can write a carboxyl group like this -CO2H.
Carboxylic acid:
– A compound containing a carboxyl, -COOH, group.
Carboxyl group:
– It is the -COOH group.
(5) Esters
– A carboxylic ester, commonly referred to as an ester, is a derivative of a carboxylic acid in which the hydrogen of the carboxyl group is replaced by a carbon-containing group.
Carboxylic ester:
– A derivative of a carboxylic acid in which H of the carboxyl group is replaced by a carbon.
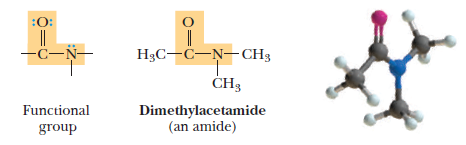
(6) Amides
– The last functional group we will explain here is A carboxylic amide, it is commonly referred to as an amide, which is a derivative of a carboxylic acid in which the -OH of the carboxyl group is replaced by an amine.
– As the model shows, the group is planar, something we will explain later.
Amide:
– A derivative of a carboxylic acid in which the -OH is replaced by an amine.
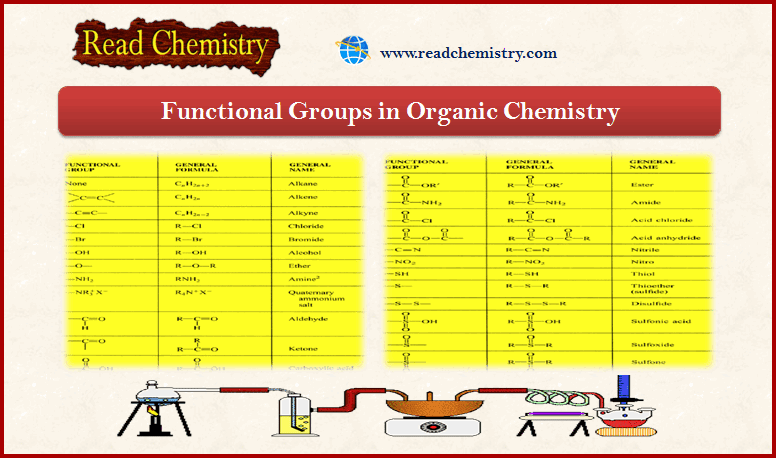
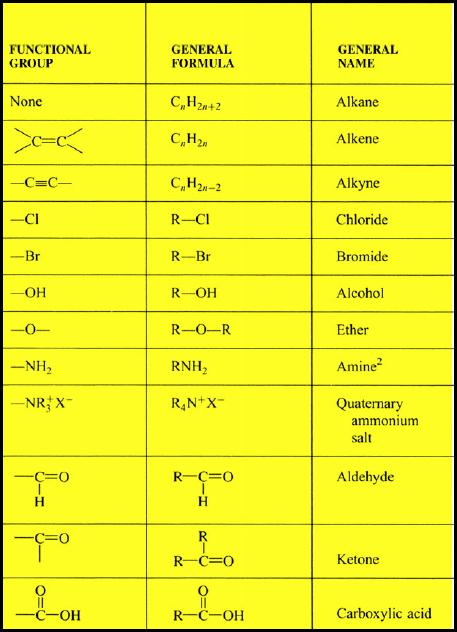
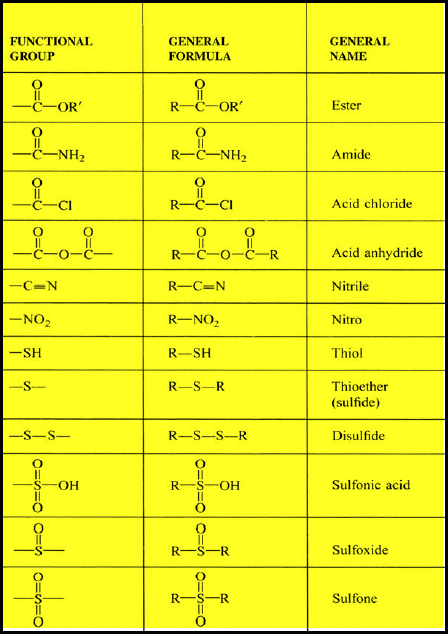
(7) Common Functional Groups Used in Organic Chemistry
– In conclusion, the table below indicates most of all Functional groups used in Organic Chemistry:
Reference: Organic chemistry / William H. Brown, Christopher S. Foote, Brent L. Iverson, Eric V. Anslyn, Bruce M. Novak. ( sixth edition) . United States.