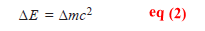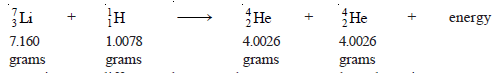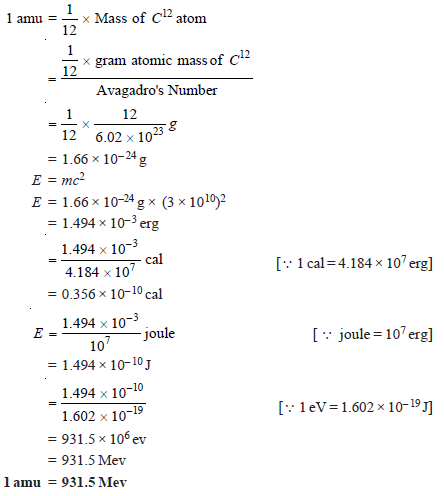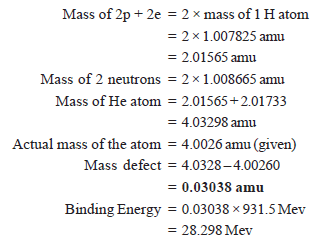Energy released in Nuclear Reactions: Nuclear Binding Energy
– In this subject, we will discuss the energy released in Nuclear Reactions: Nuclear Binding Energy
Einstein’s Equation Relating Mass and Energy
– According to Albert Einstein, mass can be converted into energy and vice versa.
– His famous equation relating mass and energy is:
where E = energy ; m = mass and c = velocity of light.
– In nuclear reactions, a change in mass, Δm, is accompanied by the release of energy, ΔE.
– Thus equation may be written as:
– If we substitute the value 3.00 × 1010 cm/sec for the velocity of light, the equation (2) directly gives the relation between the energy change in ergs and the mass change in grams:
– Making use of the conversion factor 1 erg = 2.39 × 10–11 kcal, we can express the energy change in k cals.
– Very often, in a nuclear reaction, the mass of the products is less than that of the reactants.
– The mass difference is converted into energy.
– Therefore by using equation (4), we can calculate the amount of energy released in a particular reaction.
– For example, in the equation:
– The atomic mass difference between the reactants and products is 0.0186 grams. Using equation (4):
 Mass Defect
Mass Defect
– We know that an atomic nucleus consists of protons and neutrons; collectively known as nucleons.
– It is found that the measured mass of the nucleus is always less than the sum of the masses of the individual protons and neutrons which make it up.
– Let us take the example of helium, 4He2.
– It consists of two protons and two neutrons. Its mass may be calculated as:
– However, the experimental mass of the helium nucleus is only 4.00388.
– This is less by 0.03040 amu than that calculated above.
– This is called the mass defect of the helium nucleus.
– The difference between the experimental and calculated masses of the nucleus is called the Mass defect or Mass deficit.
(Experimental mass of nucleus) – (Mass of protons + Mass of neutrons) = Mass defect
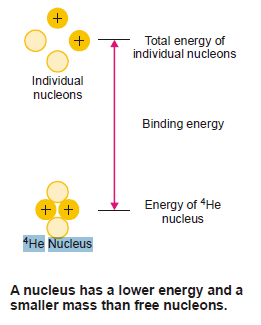
Nuclear Binding Energy
The atomic nucleus is made of protons and neutrons closely packed in a small volume.
Although there exist intensive repulsive forces between the component protons, the nucleus is not split apart.
This is so because the nucleons are bound to one another by very powerful forces.
The energy that binds the nucleons together in the nucleus is called the Nuclear binding energy.
Nuclear binding energy is The energy that binds the nucleons together in the nucleus.
When a nucleus is formed from individual protons and neutrons, there occurs a loss of mass (mass defect).
According to Einstein’s theory, it is this mass defect that is converted into binding energy.
Hence binding energy is the energy equivalent of the mass defect.
The various nuclei have different binding energies.
Binding energy is a measure of the force that holds the nucleons together.
Hence an energy equivalent to the binding energy is required to disrupt a nucleus into its constituent protons and neutrons.
Since nuclear energy is of an extremely high order, it is not easy to fission a nucleus.
Calculation of Binding Energy
The binding energies of a nucleus can be calculated from its mass defect by using Einstein’s equation, ΔE = Δm × c2.
Solved Problem (1): What is the binding energy for 11B5 nucleus if its mass defect is 0.08181 amu?
Solution:
Substituting values in Einstein’s equation,
No. of nuclei in one mole is 6.02 × 1023 (Avogadro’s Law).
∴ The binding energy for 11B5 nucleus, ΔE, may be expressed as:
Binding energy per nucleon
It can be calculated by dividing the total binding energy by the sum of the number of protons and neutrons present in the nucleus:
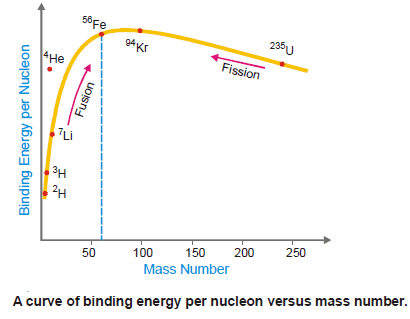
By plotting the binding energy per nucleon against the mass number, we get the graph shown in the following Figure:
The figure shows the relative stability of the various nuclei.
The greater the binding energy per nucleon the more stable the nucleus.
Thus the nuclei of about 60 atomic mass having maximum energy per nucleon are most stable e.g., 56Fe.
The nuclei that are heavier or lighter than this have lower binding energies per nucleon and are less stable.
Thus 235U undergoes fission into lighter and more stable isotopes as 139Ba and 94Kr with the release of energy.
Similarly, two or more lighter nuclei (2H, 3H) with lower binding energy per nucleon combine or fuse together into a heavier and more stable nucleus.
This is also accompanied by the release of energy.
Equivalence of amu and Energy
Since 1 amu is exactly equal to1/12 th of the mass of C12 atom, therefore
Solved Problem (2): Calculate the binding energy per nucleon (in Mev) in He atom 42 He which has a mass of 4.00260 amu. Mass of an electron = 1.008655 amu and mass of 1 hydrogen atom = 1.007825 mass.
Solution:
In a Helium atom, there are 2 electrons, 2 protons, and 2 neutrons.
1 amu = 931.5 Mev
Binding energy per nucleon:
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.