Precipitation Reactions: Definition and Examples
– In this subject, we will discuss the Precipitation Reactions: Definition and Examples
What are Precipitation Reactions?
– One common type of reaction that occurs in an aqueous solution is the precipitation reaction, which results in the formation of an insoluble product, or precipitate.
– A precipitate is an insoluble solid that separates from the solution.
– Precipitation reactions usually involve ionic compounds.
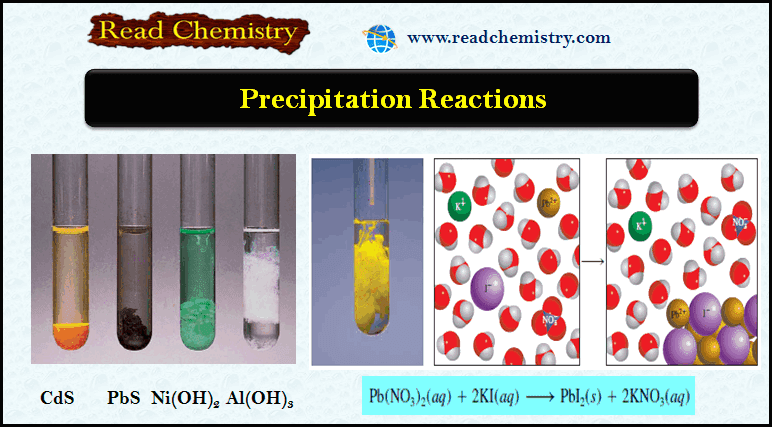
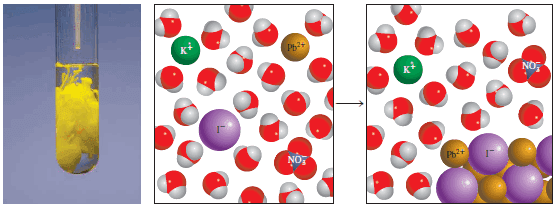
– For example, when an aqueous solution of lead (II) nitrate [Pb(NO3)2] is added to an aqueous solution of potassium iodide (KI), a yellow precipitate of lead (II) iodide (PbI2) is formed:
– Potassium nitrate remains in the solution.
– The figure below shows this reaction in progress.
– The preceding reaction is an example of a metathesis reaction (also called a double displacement reaction), a reaction that involves the exchange of parts between the two compounds.
– In this case, the cations in the two compounds exchange anions, so Pb2+ ends up with I2 as PbI2 and K+ ends up with NO3– as KNO3.
– The precipitation reactions are examples of metathesis reactions.
Solubility
– How can we predict whether a precipitate will form when a compound is added to a solution or when two solutions are mixed?
– It depends on the solubility of the solute, which is defined as the maximum amount of solute that will dissolve in a given quantity of solvent at a specific temperature.
– Chemists refer to substances as soluble, slightly soluble, or insoluble in a qualitative sense.
– A substance is said to be soluble if a fair amount of it visibly dissolves when added to water.
– If not, the substance is described as slightly soluble or insoluble.
– All ionic compounds are strong electrolytes, but they are not equally soluble.
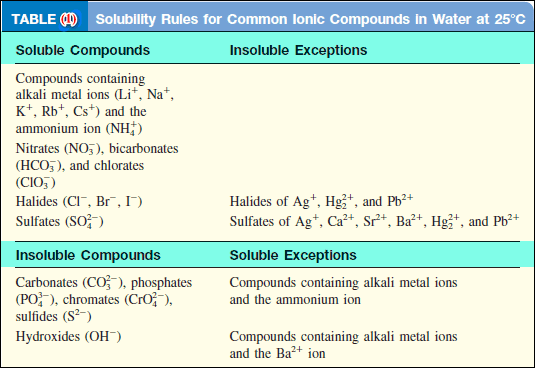
– Table (1) classifies a number of common ionic compounds as soluble or insoluble.
– Keep in mind, however, that even insoluble compounds dissolve to a certain extent.
– The figure below shows the Appearance of several precipitates.
– From left to right: CdS, PbS, Ni(OH)2, and Al(OH)3.

Example (1) on Precipitation Reactions
Classify the following ionic compounds as soluble or insoluble:
(a) Silver sulfate (Ag2 SO4 )
(b) calcium carbonate (CaCO3 )
(c) sodium phosphate (Na3 PO4 )
Strategy:
– Although it is not necessary to memorize the solubilities of compounds, you should keep in mind the following useful rules: all ionic compounds containing alkali metal cations; the ammonium ion; and the nitrate, bicarbonate, and chlorate ions are soluble.
– For other compounds, we need to refer to Table (1).
Solution:
(a) According to Table (1), Ag2 SO4 is insoluble.
(b) This is a carbonate and Ca is a Group 2A metal. Therefore, CaCO3 is insoluble.
(c) Sodium is an alkali metal (Group 1A) so Na3 PO4 is soluble.
Molecular Equations
– The equation describing the precipitation of lead (II) iodide is called a molecular equation because the formulas of the compounds are written as though all species existed as molecules or whole units.
– A molecular equation is useful because it identifies the reagents [that is, lead(II) nitrate and potassium iodide].
– If we wanted to bring about this reaction in the laboratory, we would use the molecular equation.
– However, a molecular equation does not describe in detail what actually is happening in the solution.
Ionic Equations
– when ionic compounds dissolve in water, they break apart into their component cations and anions.
– To be more realistic, the equations should show the dissociation of dissolved ionic compounds into ions.
– Therefore, returning to the reaction between potassium iodide and lead(II) nitrate, we would write:
– The preceding equation is an example of an ionic equation, which shows dissolved species as free ions.
– To see whether a precipitate might form from this solution, we first combine the cation and anion from different compounds; that is, PbI2 and KNO3.
– Referring to Table (1), we see that PbI2 is an insoluble compound and KNO3 is soluble.
– Therefore, the dissolved KNO3 remains in solution as separate K+ and NO3– ions, which are called spectator ions, or ions that are not involved in the overall reaction.
– Because spectator ions appear on both sides of an equation, they can be eliminated from the ionic equation.
Net Ionic Equations
– Finally, we end up with the net ionic equation, which shows only the species that actually take part in the reaction.
Conclusion
– Looking at another example, we find that when an aqueous solution of barium chloride (BaCl2) is added to an aqueous solution of sodium sulfate (Na2SO4), a white precipitate is formed.
– Treating this as a metathesis reaction, the products are BaSO4 and NaCl.
– From Table (1) we see that only BaSO4 is insoluble.
– Therefore, we write the molecular equation as:
– The ionic equation for the reaction is:
– Canceling the spectator ions (Na+ and Cl–) on both sides of the equation gives us the net ionic equation:
The following four steps summarize the procedure for writing ionic and net ionic equations:
(1) Write a balanced molecular equation for the reaction, using the correct formulas for the reactant and product ionic compounds.
– Refer to Table (1) to decide which of the products is insoluble and therefore will appear as a precipitate.
(2) Write the ionic equation for the reaction.
– The compound that does not appear as the precipitate should be shown as free ions.
(3) Identify and cancel the spectator ions on both sides of the equation.
– Write the net ionic equation for the reaction.
(4) Check that the charges and number of atoms balance in the net ionic equation.
– These steps are applied in Example 2
Example (2) on Precipitation Reactions
Predict what happens when a potassium phosphate (K3 PO4 ) solution is mixed with a calcium nitrate [Ca(NO3)2] solution. Write a net ionic equation for the reaction.
Strategy:
– From the given information, it is useful to first write the unbalanced equation:
– What happens when ionic compounds dissolve in water? What ions are formed from the dissociation of K3PO4 and Ca(NO3)2? and What happens when the cations encounter the anions in solution?
Solution:
– In solution, K3PO4dissociates into K+ and PO43- ions and Ca(NO3)2dissociates into Ca2+ and NO3– ions.
– According to Table (1), calcium ions (Ca2+) and phosphate ions (PO43-) will form an insoluble compound, calcium phosphate [Ca3(PO4)2], while the other product, KNO3, is soluble and remains in solution.
– Therefore, this is a precipitation reaction.
– We follow the stepwise procedure just outlined.
Step 1: The balanced molecular equation for this reaction is:
Step 2: To write the ionic equation, the soluble compounds are shown as dissociated ions:
Step3: Canceling the spectator ions (K+ and NO3–) on each side of the equation, we obtain the net ionic equation:
Step 4: Note that because we balanced the molecular equation first, the net ionic equation is balanced as to the number of atoms on each side, and the number of positive (+6) and negative (-6) charges on the left-hand side is the same.
Reference: Chemistry / Raymond Chang, Williams College /(10th edition).