Metallic Bonding: Definition, Properties, Examples, Explanation
– In this subject, we will discuss the Metallic Bonding: Definition, Properties, Examples, Explanation
Metallic Bonding
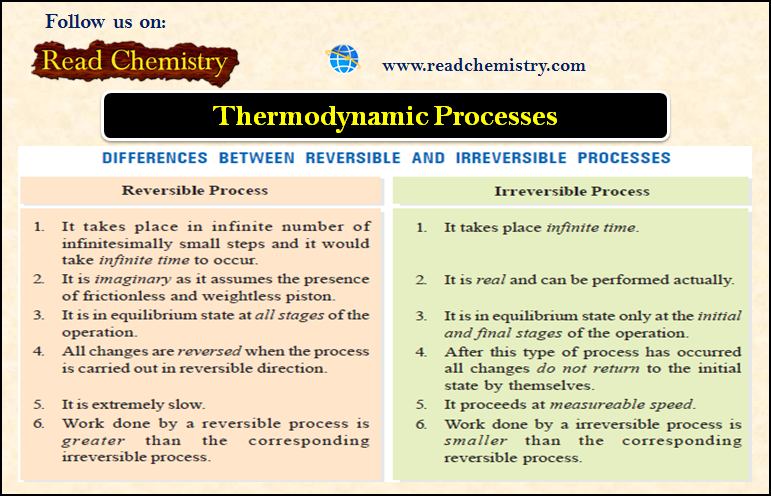
– The valence bonds that hold the atoms in a metal crystal together are not ionic, nor are they simply covalent in nature.
– Ionic bonding is obviously impossible here since all the atoms would tend to give electrons but none are willing to accept them.
– Ordinary covalent bonding is also ruled out as, for example, sodium atom with only one outer-shell electron could not be expected to form covalent bonds with 8 nearest neighbouring atoms in its crystal.
– The peculiar type of bonding that holds the atoms together in the metal crystal is called Metallic Bonding.
– Many theories have been proposed to explain the metallic bonding.
– Here we will discuss the simplest of these: The Electron Sea Model.
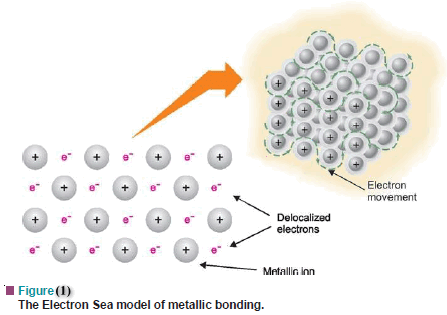
The electron Sea Model
- Metal atoms are characterized by:
– Low ionization energies which imply that the valence electrons in metal atoms can easily be separated
– Several vacant electron orbitals in their outermost shell.
– For example, the magnesium atom with the electron configuration 1s2 2s22p6 3s2 3p0 has three vacant 3p orbitals in its outer electron shell.
– There is considerable overlapping of vacant orbitals on one atom with similar orbitals of adjacent atoms, throughout the metal crystal.
– Thus an electron can be delocalized and move freely in the vacant molecular orbital encompassing the entire metal crystal. The delocalized electrons no longer belong to individual metal atoms but rather to the crystal as a whole.
– As a result of the delocalization of valence electrons, the positive metal ions that are produced, remain fixed in the crystal lattice while the delocalized electrons are free to move about in the vacant space in between.
– The metal is thus pictured as a network or lattice of positive ions of the metal immersed in a ‘sea of electrons’ or ‘gas of electrons’.
– This relatively simple model of metallic bonding is referred to as the Electron Sea model or the Electron Gas model (Fig.1)
– A metallic bond is the electrostatic force of attraction that the neighbor positive metallic ions have for the delocalized electrons.
physical properties of metals.
– The electron sea model of metallic bonding explains fairly well the most characteristic physical properties of metals.
(1) Luster or Reflectivity
– The delocalized mobile electrons of the ‘electron sea’ account for this property.
– Light energy is absorbed by these electrons which jump into higher energy levels and return immediately to the ground level.
– In doing so, the electrons emit electromagnetic radiation (light) of the same frequency.
– Since the radiated energy is of the same frequency as the incident light, we see it as a reflection of the original light.
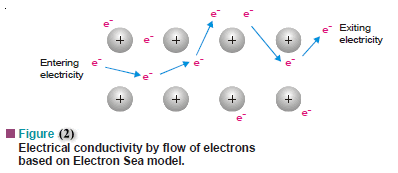
(2) Electric Conductivity
– Another characteristic of metals is that they are good conductors of electricity.
– According to the electron sea model, the mobile electrons are free to move through the vacant space between metal ions.
– When electric voltage is applied at the two ends of a metal wire, it causes the electrons to be displaced in a given direction.
– The best conductors are the metals that attract their outer electrons the least (low ionization energy) and thus allow them the greatest freedom of movement.
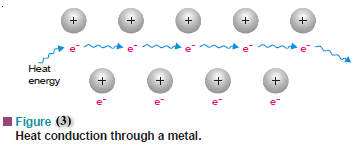
(3) Heat Conductivity
– If a metal is heated at one end, the heat is carried to the other end.
– The mobile electrons in the area of the ‘electron sea’ around one end of the metal easily absorb heat energy and increase their vibrational motion.
– They collide with adjacent electrons and transfer the added energy to them.
– Thus the mobility of the electrons allows heat transfer to the other end (Fig.3).
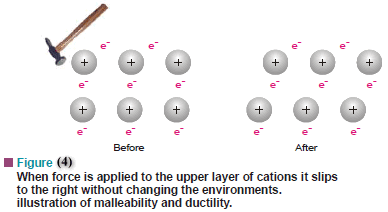
(4) Ductility and Malleability
– The ductility and malleability of metals can also be explained by the electron sea model.
– In metals, the positive ions are surrounded by the sea of electrons that ‘flows’ around them.
– If one layer of metal ions is forced across another, say by hammering, the internal structure remains essentially unchanged (Fig.4).
– The sea of electrons adjusts positions rapidly and the crystal lattice is restored.
– This allows metals to be ductile and malleable. However, in ionic crystals of salts e.g., sodium chloride, displacement of one layer of ions with respect to another brings like charged ions near to each other.
– The strong repulsive forces set up between them can cause the ionic crystals to cleave or shatter.
– Thus ionic crystals are brittle.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.