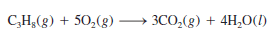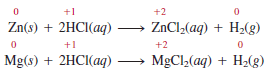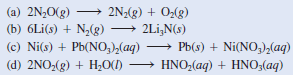Redox Reactions: Types, Examples, Applications, Balancing
– In this subject, we will discuss the Redox Reactions: Types, Examples, Applications, Balancing.
Types of Redox Reactions
– Among the most common oxidation-reduction reactions are:
- Combination Reactions
- Decomposition Reactions
- Combustion Reactions
- Displacement Reactions
- Disproportionation Reaction
(1) Combination Reactions
– A combination reaction is a reaction in which two or more substances combine to form a single product.
– Figure (1) shows some combination reactions.
– For example:
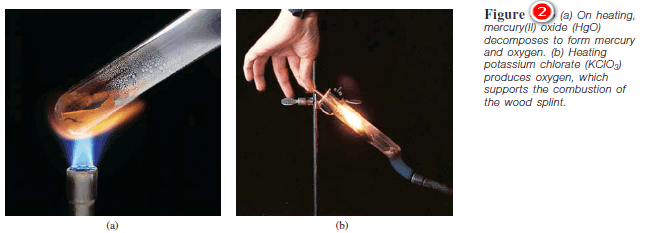
(2) Decomposition Reactions
– Decomposition reactions are the opposite of combination reactions.
– Specifically, a decomposition reaction is the breakdown of a compound into two or more components
– Figure (2) shows some combination reactions.
– For example
(3) Combustion Reactions
– A combustion reaction is a reaction in which a substance reacts with oxygen, usually with the release of heat and light to produce a flame.
– The reactions between magnesium and sulfur with oxygen described earlier are combustion reactions.
– Another example is the burning of propane (C3H8), a component of natural gas that is used for domestic heating and cooking:
– Assigning an oxidation number to C atoms in organic compounds is more involved.
– Here, we focus only on the oxidation number of O atoms, which changes from 0 to 22.
(4) Displacement Reactions
– In a displacement reaction, an ion (or atom) in a compound is replaced by an ion (or atom) of another element.
– Most displacement reactions fit into one of three subcategories: hydrogen displacement, metal displacement, or halogen displacement.
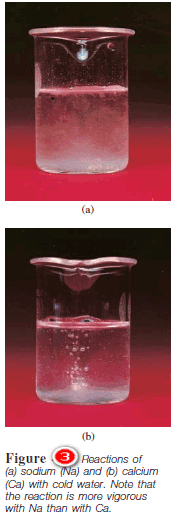
A. Hydrogen Displacement
– All alkali metals and some alkaline earth metals (Ca, Sr, and Ba), which are the most reactive of the metallic elements, will displace hydrogen from cold water ( Figure 3):
– Many metals, including those that do not react with water, are capable of displacing hydrogen from acids.
– For example, zinc (Zn) and magnesium (Mg) do not react with cold water but do react with hydrochloric acid, as follows:
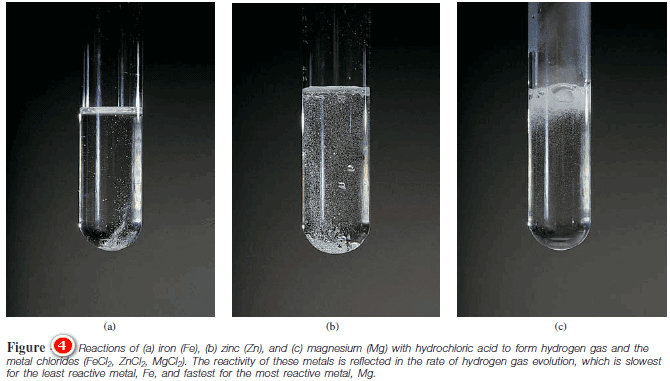
– Figure (4) shows the reactions between hydrochloric acid (HCl) and iron (Fe), zinc (Zn), and magnesium (Mg).
– These reactions are used to prepare hydrogen gas in the laboratory.
B. Metal Displacement
– A metal in a compound can be displaced by another metal in the elemental state.
– We have already seen examples of zinc replacing copper ions and copper replacing silver ions.
– Reversing the roles of the metals would result in no reaction.
– Thus, copper metal will not displace zinc ions from zinc sulfate, and silver metal will not displace copper ions from copper nitrate.
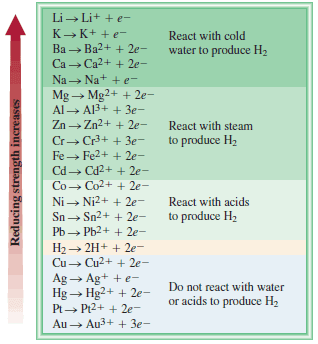
– An easy way to predict whether a metal or hydrogen displacement reaction will actually occur is to refer to an activity series (sometimes called the electrochemical series ), shown in Figure below.
– Basically, an activity series is a convenient summary of the results of many possible displacement reactions similar to the ones already discussed.
– According to this series, any metal above hydrogen will displace it from water or from an acid, but metals below hydrogen will not react with either water or an acid.
– In fact, any metal listed in the series will react with any metal (in a compound) below it.
– For example, Zn is above Cu, so zinc metal will displace copper ions from copper sulfate.
– Metal displacement reactions find many applications in metallurgical processes, the goal of which is to separate pure metals from their ores.
– For example, vanadium is obtained by treating vanadium(V) oxide with metallic calcium:
– Similarly, titanium is obtained from titanium (IV) chloride according to the reaction
– In each case, the metal that acts as the reducing agent lies above the metal that is reduced (that is, Ca is above V and Mg is above Ti) in the activity series.
C. Halogen Displacement
– Another activity series summarizes the halogens’ behavior in halogen displacement reaction:
– The power of these elements as oxidizing agents decreases as we move down Group 7A from fluorine to iodine, so molecular fluorine can replace chloride, bromide, and iodide ions in solution.
– In fact, molecular fluorine is so reactive that it also attacks water; thus these reactions cannot be carried out in aqueous solutions.
– On the other hand, molecular chlorine can displace bromide and iodide ions in aqueous solution.
– The displacement equations are:
– The ionic equations are
– Molecular bromine, in turn, can displace iodide ion in solution:
– Reversing the roles of the halogens produces no reaction.
– Thus, bromine cannot displace chloride ions, and iodine cannot displace bromide and chloride ions.
– The halogen displacement reactions have a direct industrial application.
– The halogens as a group are the most reactive of the nonmetallic elements.
– They are all strong oxidizing agents.
– As a result, they are found in nature in the combined state (with metals) as halides and never as free elements.
– Of these four elements, chlorine is by far the most important industrial chemical.
– In 2008 the amount of chlorine produced in the United States was about 25 billion pounds, making chlorine the tenth-ranking industrial chemical.
– The annual production of bromine is only one-hundredth that of chlorine, while the amounts of fluorine and iodine produced are even less.
– Recovering the halogens from their halides requires an oxidation process, which is represented by:
– where X denotes a halogen element.
– Seawater and natural brine (for example, underground water in contact with salt deposits) are rich sources of Cl2, Br2, and I2 ions.
– Minerals such as fluorite (CaF2 ) and cryolite (Na3AlF6 ) are used to prepare fluorine.
– Because fluorine is the strongest oxidizing agent known, there is no way to convert F2 ions to F2 by chemical means.
– The only way to carry out the oxidation is by electrolytic means. Industrially, chlorine, like fluorine, is produced electrolytically.
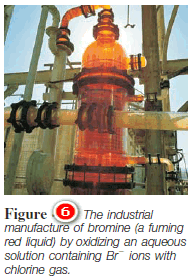
– Bromine is prepared industrially by oxidizing Br2 ions with chlorine, which is a strong enough oxidizing agent to oxidize Br2 ions but not water:
– One of the richest sources of Br2 ions is the Dead Sea—about 4000 parts per million (ppm) by mass of all dissolved substances in the Dead Sea is Br.
– Following the oxidation of Br2 ions, bromine is removed from the solution by blowing air over the solution, and the air-bromine mixture is then cooled to condense the bromine (Figure 6).
– Iodine is also prepared from seawater and natural brine by the oxidation of I2 ions with chlorine.
– Because Br2 and I2 ions are invariably present in the same source, they are both oxidized by chlorine.
– However, it is relatively easy to separate Br2 from I2 because iodine is a solid that is sparingly soluble in water.
– The air-blowing procedure will remove most of the bromine formed but will not affect the iodine present.
(5) Disproportionation Reaction
– A special type of redox reaction is the disproportionation reaction.
– In a disproportionation reaction, an element in one oxidation state is simultaneously oxidized and reduced.
– One reactant in a disproportionation reaction always contains an element that can have at least three oxidation states.
– The element itself is in an intermediate oxidation state; that is, both higher and lower oxidation states exist for that element in the products.
– The decomposition of hydrogen peroxide is an example of a disproportionation reaction.
– Here the oxidation number of oxygen in the reactant (- 1) both increases to zero in O2 and decreases to (-2) in H2O.
– Another example is the reaction between molecular chlorine and NaOH solution:
– This reaction describes the formation of household bleaching agents, for it is the hypochlorite ion (ClO2) that oxidizes the color-bearing substances in stains, converting them to colorless compounds.
– Finally, it is interesting to compare redox reactions and acid-base reactions.
– They are analogous in that acid-base reactions involve the transfer of protons while redox reactions involve the transfer of electrons.
– However, while acid-base reactions are quite easy to recognize (because they always involve an acid and a base), there is no simple procedure for identifying a redox process.
– The only sure way is to compare the oxidation numbers of all the elements in the reactants and products.
– Any change in oxidation number guarantees that the reaction is redox in nature.
– The classification of different types of redox reactions is illustrated in the following Example
Solved Example
Classify the following redox reactions and indicate changes in the oxidation numbers of the elements:
Strategy
Review the definitions of combination reactions, decomposition reactions, displacement reactions, and disproportionation reactions.
Solution:
(a) This is a decomposition reaction because one reactant is converted to two different products.
– The oxidation number of N changes from -1 to 0, while that of O changes from -2 to 0.
(b) This is a combination reaction (two reactants form a single product).
– The oxidation number of Li changes from 0 to +1 while that of N changes from 0 to -3.
(c) This is a metal displacement reaction. The Ni metal replaces (reduces) the Pb2+ion.
– The oxidation number of Ni increases from 0 to +2 while that of Pb decreases from +2 to 0.
(d) The oxidation number of N is +4 in NO2 and it is +3 in HNO2 and +5 in HNO3.
– Because the oxidation number of the same element both increases and decreases, this is a disproportionation reaction.
Reference: Chemistry / Raymond Chang, Williams College /(10th edition).