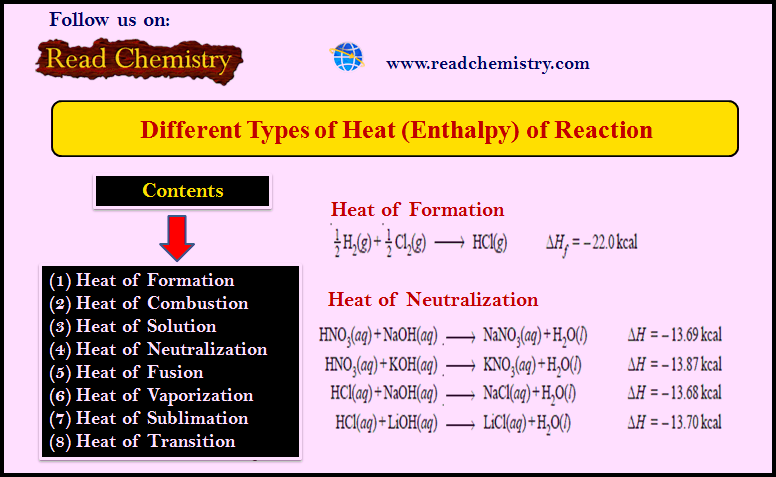
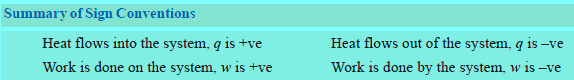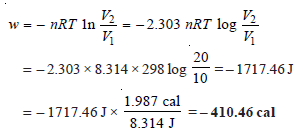Work in Thermodynamics (Definition – Formula – Problems)
– In physics, mechanical work is defined as force multiplied by the distance through which the force acts.
– In elementary thermodynamics, the only type of work generally considered is the work done in the expansion (or compression) of a gas
Nature of Heat and Work
– When a change in the state of a system occurs, energy is transferred to or from the surroundings.
– This energy may be transferred as heat or mechanical work.
– We shall refer to the term ‘work’ for mechanical work which is defined as: force × distance.
Work = force × distance
Units of Work
– In the CGS system the unit of work is erg
– Erg is the work done when a resistance of 1 dyne is moved through a distance of 1 centimeter.
– Since the erg is so small, a bigger unit, the joule (J) is now used.
1 joule = 107 ergs
1 erg = 10–7 J
– We often use kilojoules (kJ) for large quantities of work:
1 kJ = 1000 J
Units of Heat
– The unit of heat, which was used for many years, is calorie (cal).
– A calorie: is the quantity of heat required to raise the temperature of 1 gram of water by 1º C in the vicinity of 15ºC.
– Since heat and work are interrelated, the SI unit of heat is the joule (J).
1 joule = 0.2390 calories
but 1 calorie = 4.184 J
1 kcal = 4.184 kJ
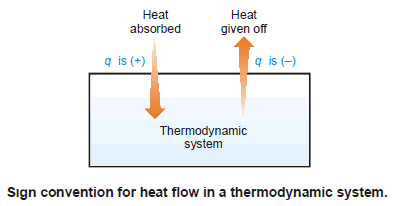
Sign Convention of Heat
– The symbol of heat is q.
– If the heat flows from the surroundings into the system to raise the energy of the system, it is taken to be positive, +q.
– If heat flows from the system into the surroundings, lowering the energy of the system, it is taken to be negative, –q.
Sign Convention of Work
– The symbol of work is w.
– If work is done on a system by the surroundings and the energy of the system is thus increased, it is taken to be positive, +w.
– If work is done by the system on the surroundings and the energy of the system is decreased, it is taken to be negative, –w.
Pressure-Volume Work
– In physics, mechanical work is defined as force multiplied by the distance through which the force acts.
– In elementary thermodynamics, the only type of work generally considered is the work done in the expansion (or compression) of a gas.
– This is known as pressure-volume work or PV work or expansion work.
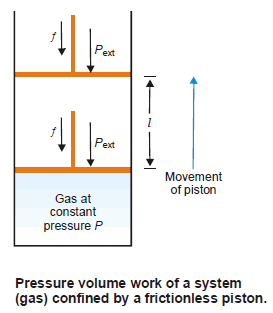
– Consider a gas contained in a cylinder fitted with a frictionless piston.
– The pressure (force per unit area) of the gas, P, exerts a force on the piston.
– This can be balanced by applying an equal but opposite pressure from outside on the piston.
Explanation of Pressure-Volume Work
– Let it be designated as Pext.
– It is important to remember that it is the external pressure, Pext. and not the internal pressure of the gas itself which is used in evaluating work.
– This is true whether it be expansion or contraction.
– If the gas expands at constant pressure, the piston would move, say through a distance l.
– We know that:
work = force × distance (by definition)
w = f × l …….(1)
– Since pressure is force per unit area,
f = Pext × A …….(2)
– where A is the cross-section area of the piston.
– From (1) and (2), we have
w = Pext × A × l
= Pext× ΔV
– where ΔV is the increase in the volume of the gas
– Since the system (gas) is doing work on the surroundings (piston), it bears a negative sign. Thus,
w = –Pext × ΔV
Proceeding as above the work done in compression of a gas can also be calculated. In that case, the piston will move down and the sign of the work will be positive.
w = Pext × ΔV
– As already stated, work may be expressed in dynes-centimeters, ergs, or joules. PV work can also be expressed as the product of pressure and volume units e.g., in litres or atmospheres.
– It may be noted that the work done by a system is not a state function.
– This is true of the mechanical work of expansion.
– We shall show presently that the work is related to the process carried out rather than to the internal and final states.
– This will be evident from a consideration of the reversible expansion and an irreversible process.
Solved Problem
Calculate the pressure-volume work done when a system containing a gas expands from 1.0 liter to 2.0 liters against a constant external pressure of 10 atmospheres. Express the answer in calories and joules.
Solution:
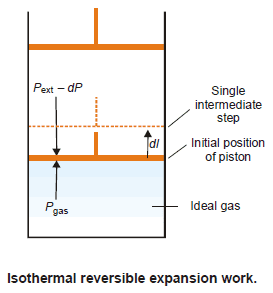
Isothermal Reversible Expansion Work of an Ideal Gas
– Consider an ideal gas confined in a cylinder with a frictionless piston.
– Suppose it expands in a reversible manner from volume V1 to V2 at a constant temperature.
– The pressure of the gas is successively reduced from P1 to P2.
– The reversible expansion of the gas takes place in a finite number of infinitesimally small intermediate steps.
– To start with the external pressure, Pext, is arranged equal to the internal pressure of the gas, Pgas, and the piston remains stationary.
– If Pext is decreased by an infinitesimal amount dP the gas expands reversibly and the piston moves through a distance dl.
– Since dP is so small, for all practical purposes,
Pext= Pgas = P
– The work done by the gas in one infinitesimal step dw, can be expressed as:
(A = cross-sectional area of piston)
dw = P × A × dl
= P × dV
– where dV is the increase in volume
– The total amount of work done by the isothermal reversible expansion of the ideal gas from V1 to V2 is, therefore:
– Isothermal compression work of an ideal gas may be derived similarly and it has exactly the same value with the sign changed.
– Here the pressure on the piston, Pext, is increased by dP which reduces the volume of the gas.
Isothermal Irreversible Expansion Work of an Ideal Gas
– Suppose we have an ideal gas contained in a cylinder with a piston.
– This time the process of expansion of the gas is performed irreversibly i.e., by instantaneously dropping the external pressure, Pext, to the final pressure P2.
– The work done by the system is now against the pressure P2 throughout the whole expansion and is given by the following expression:
Maximum Work done in Reversible Expansion
– The isothermal expansion of an ideal gas may be carried either by the reversible process or irreversible process as stated above.
– The reversible expansion is shown in the following figure in which the pressure falls as the volume increases.
– The reversible work done by the gas is given by the expression:
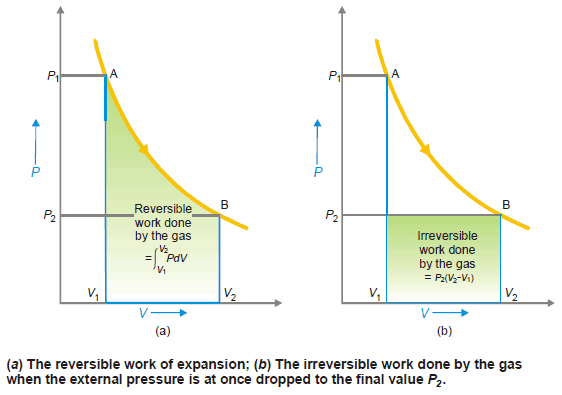
which is represented by the shaded area.
– If the expansion is performed irreversibly by suddenly reducing the external pressure to the final pressure P2, the irreversible work is given by:
– win= P2 (V2 – V1)
which is shown by the shaded area in Fig (b).
– In both the processes, the state of the system has changed from A to B but the work done is much less in the irreversible expansion than in the reversible expansion.
– Thus mechanical work is not a state function as it depends on the path by which the process is performed rather than on the initial and final states. It is a path function.
Maximum work
– It is also important to note that the work done in the reversible expansion of a gas is the maximum work that can be done by a system (gas) in expansion between the same initial (A) and final state (B).
- This is proved as follows :
– We know that the work always depends on the external pressure, Pext; the larger the Pext the more work is done by the gas.
– But the Pext on the gas cannot be more than the pressure of the gas, Pgas or compression will take place.
– Thus the largest value Pext can have without a compression taking place is equal to Pgas.
– But an expansion that occurs under these conditions is the reversible expansion.
– Thus, maximum work is done in the reversible expansion of a gas.
Solved Problem
Problem (1): One mole of an ideal gas at 25ºC is allowed to expand reversibly at constant temperature from a volume of 10 liters to 20 liters. Calculate the work done by the gas in joules and calories.
Solution:
Problem (2): Find the work done when one mole of the gas is expanded reversibly and isothermally from 5 atm to 1 atm at 25ºC.
Solution:
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.